T cell coinhibition in prostate cancer: new immune evasion pathways and emerging therapeutics
- PMID: 20971039
- PMCID: PMC3039708
- DOI: 10.1016/j.molmed.2010.09.006
T cell coinhibition in prostate cancer: new immune evasion pathways and emerging therapeutics
Abstract
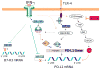
T cell-mediated adaptive immune response is controlled by both positive costimulation and negative coinhibition, generated mainly by the interaction between the B7 family and their receptor CD28 family. Coinhibition is exploited by prostate cancer as an immune evasion pathway. Overexpression of coinhibitory B7x and B7-H3 in prostate cancer correlates with poor disease outcome, whereas tumor-infiltrating immune cells have enhanced expression of PD-L1 and its receptor PD-1. New insights into the complex mechanisms governing B7 expression in the tumor microenvironment have been reported and therapies aimed at overcoming T cell coinhibition with antagonistic monoclonal antibodies are emerging as effective tumor immunotherapies. Therapies that block B7x and B7-H3, either as monotherapies or in synergism with traditional therapies, should be pursued.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Ferlay J, et al. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- Riegman PH, et al. Characterization of the prostate-specific antigen gene: a novel human kallikrein-like gene. Biochem Biophys Res Commun. 1989;159:95–102. - PubMed
-
- Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–2324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

