A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo
- PMID: 20971185
- PMCID: PMC3014388
- DOI: 10.1016/j.freeradbiomed.2010.10.696
A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo
Abstract
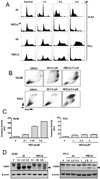
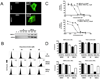
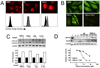
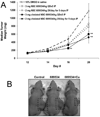
In this study, a Cu(2+) chelate of the novel thiosemicarbazone NSC 689534 was evaluated for in vitro and in vivo anti-cancer activity. Results demonstrated that NSC 689534 activity (low micromolar range) was enhanced four- to fivefold by copper chelation and completely attenuated by iron. Importantly, once formed, the NSC 689534/Cu(2+) complex retained activity in the presence of additional iron or iron-containing biomolecules. NSC 689534/Cu(2+) mediated its effects primarily through the induction of ROS, with depletion of cellular glutathione and protein thiols. Pretreatment of cells with the antioxidant N-acetyl-l-cysteine impaired activity, whereas NSC 689534/Cu(2+) effectively synergized with the glutathione biosynthesis inhibitor buthionine sulfoximine. Microarray analysis of NSC 689534/Cu(2+)-treated cells highlighted activation of pathways involved in oxidative and ER stress/UPR, autophagy, and metal metabolism. Further scrutiny of the role of ER stress and autophagy indicated that NSC 689534/Cu(2+)-induced cell death was ER-stress dependent and autophagy independent. Last, NSC 689534/Cu(2+) was shown to have activity in an HL60 xenograft model. These data suggest that NSC 689534/Cu(2+) is a potent oxidative stress inducer worthy of further preclinical investigation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Calcium release induced by 2-pyridinecarboxaldehyde thiosemicarbazone and its copper complex contributes to tumor cell death.Oncol Rep. 2017 Mar;37(3):1662-1670. doi: 10.3892/or.2017.5395. Epub 2017 Jan 20. Oncol Rep. 2017. PMID: 28112358
-
Metformin induces an intracellular reductive state that protects oesophageal squamous cell carcinoma cells against cisplatin but not copper-bis(thiosemicarbazones).BMC Cancer. 2014 May 5;14:314. doi: 10.1186/1471-2407-14-314. BMC Cancer. 2014. PMID: 24886082 Free PMC article.
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
The unfolded protein response as a target for anticancer therapeutics.Crit Rev Oncol Hematol. 2018 Jul;127:66-79. doi: 10.1016/j.critrevonc.2018.05.003. Epub 2018 May 26. Crit Rev Oncol Hematol. 2018. PMID: 29891114 Review.
-
Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review.
Cited by
-
Synthesis, Characterization and Biological Activity of Novel Cu(II) Complexes of 6-Methyl-2-Oxo-1,2-Dihydroquinoline-3-Carbaldehyde-4n-Substituted Thiosemicarbazones.Molecules. 2020 Apr 17;25(8):1868. doi: 10.3390/molecules25081868. Molecules. 2020. PMID: 32316698 Free PMC article.
-
Perspective: Opportunities in recalcitrant, rare and neglected tumors.Oncol Rep. 2013 Sep;30(3):1030-4. doi: 10.3892/or.2013.2581. Epub 2013 Jul 2. Oncol Rep. 2013. PMID: 23820887 Free PMC article.
-
Oxyphenisatin acetate (NSC 59687) triggers a cell starvation response leading to autophagy, mitochondrial dysfunction, and autocrine TNFα-mediated apoptosis.Cancer Med. 2013 Oct;2(5):687-700. doi: 10.1002/cam4.107. Epub 2013 Jul 23. Cancer Med. 2013. PMID: 24403234 Free PMC article.
-
Development and Validation of a Prognostic Classifier Based on Lipid Metabolism-Related Genes for Breast Cancer.J Inflamm Res. 2022 Jun 14;15:3477-3499. doi: 10.2147/JIR.S357144. eCollection 2022. J Inflamm Res. 2022. PMID: 35726216 Free PMC article.
-
Copper Coordination Compounds as Biologically Active Agents.Int J Mol Sci. 2020 May 31;21(11):3965. doi: 10.3390/ijms21113965. Int J Mol Sci. 2020. PMID: 32486510 Free PMC article. Review.
References
-
- Yu Y, Kalinowski DS, Kovacevic Z, Siafakas AR, Jansson PJ, Stefani C, Lovejoy DB, Sharpe PC, Bernhardt PV, Richardson DR. Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductase inhibitors. J Med Chem. 2009;52:5271–5294. - PubMed
-
- Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res. 2006;12:6876–6883. - PubMed
-
- Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. J Clin Oncol. 2004;22:1553–1563. - PubMed
-
- Shao J, Zhou B, Zhu L, Qiu W, Yuan YC, Xi B, Yen Y. In vitro characterization of enzymatic properties and inhibition of the p53R2 subunit of human ribonucleotide reductase. Cancer Res. 2004;64:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

