Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers
- PMID: 20971506
- PMCID: PMC2993249
- DOI: 10.1016/j.biomaterials.2010.10.003
Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers
Abstract
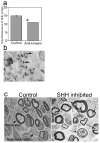
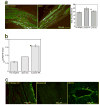
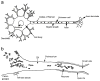
SHH plays a significant role in peripheral nerve regeneration and has clinical potential to be used as a regenerative therapy for the CN in prostatectomy patients and in other patients with neuropathy of peripheral nerves. Efforts to regenerate the cavernous nerve (CN), which provides innervation to the penis, have been minimally successful, with little translation into improved clinical outcomes. We propose that, Sonic hedgehog (SHH), is critical to maintain CN integrity, and that SHH delivered to the CN by novel peptide amphiphile (PA) nanofibers, will promote CN regeneration, restore physiological function, and prevent penile morphology changes that result in erectile dysfunction (ED). We performed localization studies, inhibition of SHH signaling in the CN, and treatment of crushed CNs with SHH protein via linear PA gels, which are an innovative extended release method of delivery. Morphological, functional and molecular analysis revealed that SHH protein is essential to maintain CN architecture, and that SHH treatment promoted CN regeneration, suppressed penile apoptosis and caused a 58% improvement in erectile function in less than half the time reported in the literature. These studies show that SHH has substantial clinical application to regenerate the CN in prostatectomy and diabetic patients, that this methodology has broad application to regenerate any peripheral nerve that SHH is necessary for maintenance of its structure, and that this nanotechnology method of protein delivery may have wide spread application as an in vivo delivery tool in many organs.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures




References
-
- Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, et al. Association between erectile dysfunction and coronary artery disease: matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–731. - PubMed
-
- User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. - PubMed
-
- Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. - PubMed
-
- Perimenis P, Markou S, Gyftopoulos K, Athanasopoulos A, Giannitsas K, Barbalias G. Switching from long-term treatment with self-injections to oral sildenafil in diabetic patients with severe erectile dysfunction. Eur Urol. 2002;41:387–391. - PubMed
-
- Connolly SS, Yoo JJ, Abouheba M, Soker S, McDougal WS, Atala A. Cavernous nerve regeneration using acellular nerve grafts. World J Urol. 2008;26:333–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

