4-[1-(Substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzenesulfonic acids: synthesis, antimicrobial activity, QSAR studies, and antiviral evaluation
- PMID: 20971531
- PMCID: PMC7115694
- DOI: 10.1016/j.ejmech.2010.09.065
4-[1-(Substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzenesulfonic acids: synthesis, antimicrobial activity, QSAR studies, and antiviral evaluation
Abstract
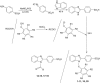
A series of 4-[1-(substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzene sulphonic acids (1-20) was synthesized and evaluated, in vitro, for their antimicrobial activity and the results indicated that compounds 4-[1-(4-Nitrobenzoyl)-1H-benzoimidazol-2-yl]-benzenesulfonic acid (9) and 4-(1-octadec-9-enoyl-1H-benzoimidazol-2-yl)-benzenesulfonic acid (18) were found to be the most active ones. QSAR investigations indicated that the multi-target QSAR model was effective in describing the antimicrobial activity over the one-target QSAR models. Further the mt-QSAR model indicated the importance of the topological parameter, Balaban index (J) followed by the electronic parameter, LUMO and topological parameter, valence second order molecular connectivity index (2χv) in describing the antimicrobial activity of synthesized compounds (1-20).
Copyright © 2010 Elsevier Masson SAS. All rights reserved.
Figures




Similar articles
-
Benzylidene/2-chlorobenzylidene hydrazides: synthesis, antimicrobial activity, QSAR studies and antiviral evaluation.Eur J Med Chem. 2010 Jul;45(7):2806-16. doi: 10.1016/j.ejmech.2010.03.002. Epub 2010 Mar 7. Eur J Med Chem. 2010. PMID: 20347509 Free PMC article.
-
Synthesis and QSAR evaluation of 2-(substituted phenyl)-1H-benzimidazoles and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones.Eur J Med Chem. 2009 Mar;44(3):1119-27. doi: 10.1016/j.ejmech.2008.06.009. Epub 2008 Jun 24. Eur J Med Chem. 2009. PMID: 18657885
-
Synthesis, antimicrobial and antiviral activity of substituted benzimidazoles.J Enzyme Inhib Med Chem. 2009 Oct;24(5):1161-8. doi: 10.1080/14756360802694427. J Enzyme Inhib Med Chem. 2009. PMID: 19772489
-
Synthesis, antimicrobial evaluation and QSAR studies of 2-hydroxy propanoic acid derivatives.Drug Res (Stuttg). 2014 May;64(5):240-5. doi: 10.1055/s-0033-1357127. Epub 2013 Oct 8. Drug Res (Stuttg). 2014. PMID: 24105102
-
Hybrid Compounds as Anti-infective Agents.Curr Top Med Chem. 2017;17(9):1080-1095. doi: 10.2174/1568026616666160927160912. Curr Top Med Chem. 2017. PMID: 27697047 Review.
Cited by
-
Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives.Molecules. 2015 Sep 10;20(9):16419-34. doi: 10.3390/molecules200916419. Molecules. 2015. PMID: 26378507 Free PMC article.
-
Enhanced thermal stability, hydrophobicity, UV radiation resistance, and antibacterial properties of wool fabric treated with p-aminobenzenesulphonic acid.RSC Adv. 2020 May 5;10(30):17515-17523. doi: 10.1039/d0ra02267e. eCollection 2020 May 5. RSC Adv. 2020. PMID: 35515614 Free PMC article.
-
Antimicrobial potential of 1H-benzo[d]imidazole scaffold: a review.BMC Chem. 2019 Feb 4;13(1):18. doi: 10.1186/s13065-019-0521-y. eCollection 2019 Dec. BMC Chem. 2019. PMID: 31384767 Free PMC article. Review.
-
Pyridine Compounds with Antimicrobial and Antiviral Activities.Int J Mol Sci. 2022 May 18;23(10):5659. doi: 10.3390/ijms23105659. Int J Mol Sci. 2022. PMID: 35628466 Free PMC article. Review.
-
Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides.Chem Cent J. 2018 May 26;12(1):66. doi: 10.1186/s13065-018-0432-3. Chem Cent J. 2018. PMID: 29804151 Free PMC article.
References
-
- Saleh M., Abbott S., Lauzon V.C., Penney C., Zacharie B. Bioorg. Med. Chem. Lett. 2010;20:945–949. - PubMed
-
- Miller J.F., Turner E.M., Gudmundsson K.S., Jenkinson S., Spaltenstein A., Thomson M., Wheelan P. Bioorg. Med. Chem. Lett. 2010;20:2125–2128. - PubMed
-
- Tonelli M., Simone M., Tasso B., Novelli F., Boido V., Sparatore F., Paglietti G., Pricl S., Giliberti G., Blois S., Ibba C., Sanna G., Loddo R., Colla P.L. Bioorg. Med. Chem. 2010;18:2937–2953. - PubMed
-
- Luo X., Zhang Z., Yang Y., Xue F., Xiu N., She Y. Front. Chem. Eng. China. 2009;3(3):305–309.
-
- Pilyugin V.S., Kuznetsova S.L., Sapozhnikov Y.E., Chikisheva G.E., Kiseleva G.V., Vorob’eva T.P., Klimakova E.V., Sapozhnikova N.A., Davletov R.D., Galeeva Z.B. Russ. J. Gen. Chem. 2008;78(3):446–450.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

