Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation
- PMID: 20971705
- PMCID: PMC2964108
- DOI: 10.1242/jcs.067645
Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation
Abstract
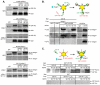
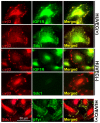
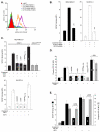
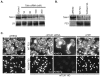
Syndecan-1 (Sdc1) engages and activates the αvβ3 (and/or αvβ5) integrin when clustered in human carcinoma and endothelial cells. Although the engagement is extracellular, the activation mechanism is cytoplasmic. This talin-dependent, inside-out signaling pathway is activated downstream of the insulin-like growth factor-1 receptor (IGF1R), whose kinase activity is triggered by Sdc1 clustering. In vitro binding assays using purified receptors suggest that association of the Sdc1 ectodomain with the integrin provides a 'docking face' for IGF1R. IGF1R docking and activation of the associated integrin is blocked by synstatin (SSTN(92-119)), a peptide derived from the integrin engagement site in Sdc1. IGF1R colocalizes with αvβ3 integrin and Sdc1 in focal contacts, but fails to associate with or activate the integrin in cells either lacking Sdc1 or expressing Sdc1(Δ67-121), a mutant that is unable to form the Sdc1-integrin-IGF1R ternary complex. Integrin activation is also blocked by IGF1R inhibitors or by silencing IGF1R or talin expression with small-interfering RNAs (siRNAs). In both cases, expression of the constitutively active talin F23 head domain rescues integrin activation. We recently reported that SSTN(92-119) blocks angiogenesis and impairs tumor growth in mice, therefore this Sdc1-mediated integrin regulatory mechanism might be a crucial regulator of disease processes known to rely on these integrins, including tumor cell metastasis and tumor-induced angiogenesis.
Figures



References
-
- Alexopoulou A. N., Multhaupt H. A., Couchman J. R. (2007). Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 39, 505-528 - PubMed
-
- Arnaout M. A., Mahalingam B., Xiong J. P. (2005). Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21, 381-410 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

