Matriptase is involved in ErbB-2-induced prostate cancer cell invasion
- PMID: 20971737
- PMCID: PMC2993294
- DOI: 10.2353/ajpath.2010.100228
Matriptase is involved in ErbB-2-induced prostate cancer cell invasion
Abstract
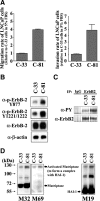
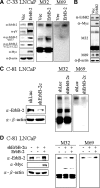
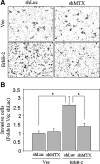
Deregulation of both ErbB-2 signaling and matriptase activity has been associated with human prostate cancer (PCa) progression. In this communication, we investigated the roles of both ErbB-2 signaling in matriptase zymogen activation and matriptase in ErbB-2-induced PCa malignancy. In a human PCa cell progression model, we observed that advanced PCa C-81 LNCaP cells exhibited an aggressive phenotype with increased cell migration and invasion capacity; these cells concurrently showed both enhanced ErbB-2 phosphorylation and increased matriptase zymogen activation compared with parental C-33 LNCaP cells. Moreover, ErbB2 activation, both ligand-dependent (eg, epidermal growth factor treatment) and ligand-independent (eg, overexpression), was able to induce matriptase zymogen activation in this cell line. Inhibition of ErbB-2 activity by either the specific inhibitor, AG825, in epidermal growth factor-treated C-33 LNCaP cells or ErbB-2 knockdown in C-81 LNCaP cells, reduced matriptase activation. These observations were confirmed by similar studies using both DU145 and PC3 cells. Together, these data suggest that ErbB-2 signaling plays an important role in matriptase zymogen activation. ErbB-2-enhanced matriptase activation was suppressed by a phosphatidylinositol 3-kinase inhibitor (ie, LY294002) but not by a MEK inhibitor (ie, PD98059). Suppression of matriptase expression by small hairpin RNA knockdown in ErbB-2-overexpressing LNCaP cells dramatically suppressed cancer cell invasion. In summary, our data indicate that ErbB-2 signaling via the phosphatidylinositol 3-kinase pathway results in up-regulated matriptase zymogen activity, which contributes to PCa cell invasion.
Figures




References
-
- Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32 Suppl:S66–S81. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. - PubMed
-
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. - PubMed
-
- Carles J, Lloreta J, Salido M, Font A, Suarez M, Baena V, Nogue M, Domenech M, Fabregat X. Her-2/neu expression in prostate cancer: a dynamic process? Clin Cancer Res. 2004;10:4742–4745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

