Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits
- PMID: 20971764
- PMCID: PMC3023244
- DOI: 10.1152/ajpheart.00857.2010
Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits
Abstract
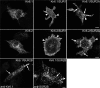
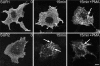
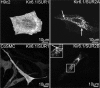
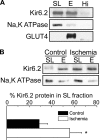
ATP-sensitive K(+) (K(ATP)) channels, composed of inward rectifier K(+) (Kir)6.x and sulfonylurea receptor (SUR)x subunits, are expressed on cellular plasma membranes. We demonstrate an essential role for SUR2 subunits in trafficking K(ATP) channels to an intracellular vesicular compartment. Transfection of Kir6.x/SUR2 subunits into a variety of cell lines (including h9c2 cardiac cells and human coronary artery smooth muscle cells) resulted in trafficking to endosomal/lysosomal compartments, as assessed by immunofluorescence microscopy. By contrast, SUR1/Kir6.x channels efficiently localized to the plasmalemma. The channel turnover rate was similar with SUR1 or SUR2, suggesting that the expression of Kir6/SUR2 proteins in lysosomes is not associated with increased degradation. Surface labeling of hemagglutinin-tagged channels demonstrated that SUR2-containing channels dynamically cycle between endosomal and plasmalemmal compartments. In addition, Kir6.2 and SUR2 subunits were found in both endosomal and sarcolemmal membrane fractions isolated from rat hearts. The balance of these K(ATP) channel subunits shifted to the sarcolemmal membrane fraction after the induction of ischemia. The K(ATP) channel current density was also increased in rat ventricular myocytes isolated from hearts rendered ischemic before cell isolation without corresponding changes in subunit mRNA expression. We conclude that an intracellular pool of SUR2-containing K(ATP) channels exists that is derived by endocytosis from the plasma membrane. In cardiac myocytes, this pool can potentially play a cardioprotective role by serving as a reservoir for modulating surface K(ATP) channel density under stress conditions, such as myocardial ischemia.
Figures


Comment in
-
Differential roles for SUR subunits in KATP channel membrane targeting and regulation.Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H33-5. doi: 10.1152/ajpheart.01088.2010. Epub 2010 Nov 5. Am J Physiol Heart Circ Physiol. 2011. PMID: 21057044 Free PMC article. No abstract available.
References
-
- Aguilar-Bryan L, Bryan J, Nakazaki M. Of mice and men: K(ATP) channels and insulin secretion. Recent Prog Horm Res 56:47–68, 2001 - PubMed
-
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60:667–687, 1998 - PubMed
-
- Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes 45:1439–1445, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

