The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia
- PMID: 20971803
- PMCID: PMC3023285
- DOI: 10.1152/ajplung.00256.2010
The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia
Abstract
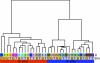
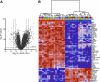
Organotypic cultures of primary human airway epithelial cells have been used to investigate the morphology, ion and fluid transport, innate immunity, transcytosis, infection, inflammation, signaling, cilia, and repair functions of this complex tissue. However, we do not know how closely these cultures resemble the airway surface epithelium in vivo. In this study, we examined the genome-wide expression profile of tracheal and bronchial human airway epithelia in vivo and compared it with the expression profile of primary cultures of human airway epithelia grown at the air-liquid interface. For comparison, we also investigated the expression profile of Calu-3 cells grown at the air-liquid interface and primary cultures of human airway epithelia submerged in nutrient media. We found that the transcriptional profile of differentiated primary cultures grown at the air-liquid interface most closely resembles that of in vivo airway epithelia, suggesting that the use of primary cultures and the presence of an air-liquid interface are important to recapitulate airway epithelia biology. We describe a high level of similarity between cells of tracheal and bronchial origin within and between different human donors, which suggests a very robust expression profile that is specific to airway cells.
Figures


References
-
- Clarke S, Gordon S. Myeloid-specific gene expression. J Leukoc Biol 63: 153–168, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

