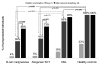Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host
- PMID: 20971824
- PMCID: PMC3031700
- DOI: 10.3324/haematol.2010.032664
Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host
Abstract
Background: In 2009 the declaration by the World Health Organization of a global pandemic of influenza-H1N1 virus led to a vaccination campaign to ensure protection for immunocompromised patients. The goal of this study was to determine the efficacy of the 2009 H1N1 vaccine in patients with hematologic malignancies.
Design and methods: We evaluated humoral and cellular immune responses to 2009 H1N1 vaccine in 97 adults with hematologic malignancies and compared these responses with those in 25 adult controls. Patients received two injections of vaccine 21 days apart and the controls received one dose. Antibody titers were measured using a hemagglutination-inhibition assay on days 0, 21 and 49 after injection of the first dose. Cellular immune responses to H1N1 were determined on days 0 and 49.
Results: By day 21 post-vaccination, protective antibody titers of 1:32 or more were seen in 100% of controls compared to 39% of patients with B-cell malignancies (P<0.001), 46% of allogeneic stem cell transplant recipients (P<0.001) and 85% of patients with chronic myeloid leukemia (P=0.086). After a second dose, seroprotection rates increased to 68%, (P=0.008), 73%, (P=0.031), and 95% (P=0.5) in patients with B-cell malignancies, after allogeneic stem cell transplantation and with chronic myeloid leukemia, respectively. On the other hand, T-cell responses to H1N1 vaccine were not significantly different between patients and controls.
Conclusions: These data demonstrate the efficacy of H1N1 vaccine in most patients with hematologic malignancies and support the recommendation for the administration of two doses of vaccine in immunocompromised patients. These results may contribute towards the development of evidence-based guidelines for influenza vaccination in such patients in the future.
Figures
References
-
- The European Agency for the Evaluation of Medicinal Products (EMEA) European Medicines Agency recommends authorisation of two vaccines for influenza pandemic (H1N1) 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/....
-
- Department of Health. H1N1 swine flu vaccination programme 2009–2010. 2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/De....
-
- Pollyea DA, Brown JM, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28(14):2481–90. - PubMed
-
- Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–8. - PubMed
-
- The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Proprietary Medicinal Products (CPMP) Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. 1-3-1997. http://www.emea.europa.eu/pdfs/human/bwp/021496en.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical