The rate of folding dictates substrate secretion by the Escherichia coli hemolysin type 1 secretion system
- PMID: 20971850
- PMCID: PMC3003356
- DOI: 10.1074/jbc.M110.173658
The rate of folding dictates substrate secretion by the Escherichia coli hemolysin type 1 secretion system
Abstract
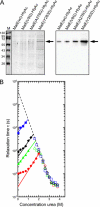
Secretion of the Escherichia coli toxin hemolysin A (HlyA) is catalyzed by the membrane protein complex HlyB-HlyD-TolC and requires a secretion sequence located within the last 60 amino acids of HlyA. The Hly translocator complex exports a variety of passenger proteins when fused N-terminal to this secretion sequence. However, not all fusions are secreted efficiently. Here, we demonstrate that the maltose binding protein (MalE) lacking its natural export signal and fused to the HlyA secretion signal is poorly secreted by the Hly system. We anticipated that folding kinetics might be limiting secretion, and we therefore introduced the "folding" mutation Y283D. Indeed this mutant fusion protein was secreted at a much higher level. This level was further enhanced by the introduction of a second MalE folding mutation (V8G or A276G). Secretion did not require the molecular chaperone SecB. Folding analysis revealed that all mutations reduced the refolding rate of the substrate, whereas the unfolding rate was unaffected. Thus, the efficiency of secretion by the Hly system is dictated by the folding rate of the substrate. Moreover, we demonstrate that fusion proteins defective in export can be engineered for secretion while still retaining function.
Figures

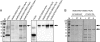

References
-
- Holland I. B., Schmitt L., Young J. (2005) Mol. Membr. Biol. 22, 29–39 - PubMed
-
- Wickner W., Schekman R. (2005) Science 310, 1452–1456 - PubMed
-
- Davidson A. L., Chen J. (2004) Annu. Rev. Biochem. 73, 241–268 - PubMed
-
- Koronakis V., Sharff A., Koronakis E., Luisi B., Hughes C. (2000) Nature 405, 914–919 - PubMed
-
- Johnson J. M., Church G. M. (1999) J. Mol. Biol. 287, 695–715 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

