Mapping interactions between germinants and Clostridium difficile spores
- PMID: 20971909
- PMCID: PMC3019946
- DOI: 10.1128/JB.00980-10
Mapping interactions between germinants and Clostridium difficile spores
Abstract
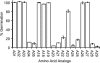
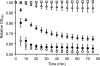
Germination of Clostridium difficile spores is the first required step in establishing C. difficile-associated disease (CDAD). Taurocholate (a bile salt) and glycine (an amino acid) have been shown to be important germinants of C. difficile spores. In the present study, we tested a series of glycine and taurocholate analogs for the ability to induce or inhibit C. difficile spore germination. Testing of glycine analogs revealed that both the carboxy and amino groups are important epitopes for recognition and that the glycine binding site can accommodate compounds with more widely separated termini. The C. difficile germination machinery also recognizes other hydrophobic amino acids. In general, linear alkyl side chains are better activators of spore germination than their branched analogs. However, L-phenylalanine and L-arginine are also good germinants and are probably recognized by distinct binding sites. Testing of taurocholate analogs revealed that the 12-hydroxyl group of taurocholate is necessary, but not sufficient, to activate spore germination. In contrast, the 6- and 7-hydroxyl groups are required for inhibition of C. difficile spore germination. Similarly, C. difficile spores are able to detect taurocholate analogs with shorter, but not longer, alkyl amino sulfonic acid side chains. Furthermore, the sulfonic acid group can be partially substituted with other acidic groups. Finally, a taurocholate analog with an m-aminobenzenesulfonic acid side chain is a strong inhibitor of C. difficile spore germination. In conclusion, C. difficile spores recognize both amino acids and taurocholate through multiple interactions that are required to bind the germinants and/or activate the germination machinery.
Figures
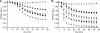


Similar articles
-
Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores.J Bacteriol. 2010 Aug;192(16):4215-22. doi: 10.1128/JB.00488-10. Epub 2010 Jun 18. J Bacteriol. 2010. PMID: 20562307 Free PMC article.
-
Bile salts and glycine as cogerminants for Clostridium difficile spores.J Bacteriol. 2008 Apr;190(7):2505-12. doi: 10.1128/JB.01765-07. Epub 2008 Feb 1. J Bacteriol. 2008. PMID: 18245298 Free PMC article.
-
Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination.J Bacteriol. 2009 Feb;191(3):1115-7. doi: 10.1128/JB.01260-08. Epub 2008 Dec 5. J Bacteriol. 2009. PMID: 19060152 Free PMC article.
-
Bile acids as germinants for Clostridioides difficile spores, evidence of adaptation to the gut?FEMS Microbiol Rev. 2025 Jan 14;49:fuaf005. doi: 10.1093/femsre/fuaf005. FEMS Microbiol Rev. 2025. PMID: 39924167 Free PMC article. Review.
-
A Revised Understanding of Clostridioides difficile Spore Germination.Trends Microbiol. 2020 Sep;28(9):744-752. doi: 10.1016/j.tim.2020.03.004. Epub 2020 Apr 23. Trends Microbiol. 2020. PMID: 32781028 Review.
Cited by
-
Regulation of Clostridium difficile spore germination by the CspA pseudoprotease domain.Biochimie. 2016 Mar;122:243-54. doi: 10.1016/j.biochi.2015.07.023. Epub 2015 Jul 29. Biochimie. 2016. PMID: 26231446 Free PMC article.
-
Conservation of the "Outside-in" Germination Pathway in Paraclostridium bifermentans.Front Microbiol. 2018 Oct 17;9:2487. doi: 10.3389/fmicb.2018.02487. eCollection 2018. Front Microbiol. 2018. PMID: 30386321 Free PMC article.
-
An Aniline-Substituted Bile Salt Analog Protects both Mice and Hamsters from Multiple Clostridioides difficile Strains.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0143521. doi: 10.1128/AAC.01435-21. Epub 2021 Nov 15. Antimicrob Agents Chemother. 2022. PMID: 34780262 Free PMC article.
-
Characterization of the Dynamic Germination of Individual Clostridium difficile Spores Using Raman Spectroscopy and Differential Interference Contrast Microscopy.J Bacteriol. 2015 Jul;197(14):2361-73. doi: 10.1128/JB.00200-15. Epub 2015 May 4. J Bacteriol. 2015. PMID: 25939833 Free PMC article.
-
Requirements for in vitro germination of Paenibacillus larvae spores.J Bacteriol. 2013 Mar;195(5):1005-11. doi: 10.1128/JB.01958-12. Epub 2012 Dec 21. J Bacteriol. 2013. PMID: 23264573 Free PMC article.
References
-
- Abel-Santos, E., and T. Dodatko. 2007. Differential nucleoside recognition during Bacillus cereus 569 (ATCC 10876) spore germination. New J. Chem. 31:748-755.
-
- Arslan, H., E. K. Inci, O. K. Azap, H. Karakayali, A. Torgay, and M. Haberal. 2007. Etiologic agents of diarrhea in solid organ recipients. Transplant Infect. Dis. 9:270-275. - PubMed
-
- Bandyopadhyay, P., V. Janout, L. H. Zhang, and S. L. Regen. 2001. Ion conductors derived from cholic acid and spermine: importance of facial hydrophilicity on Na+ transport and membrane selectivity. J. Am. Chem. Soc. 123:7691-7696. - PubMed
-
- Bartlett, J. G. 2007. Clostridium difficile: old and new observations. J. Clin. Gastroenterol. 41:S24-S29.
-
- Cloud, J., and C. P. Kelly. 2007. Update on Clostridium difficile associated disease. Curr. Opin. Gastroenterol. 23:4-9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

