Store-operated Ca²+ signaling in dendritic cells occurs independently of STIM1
- PMID: 20971921
- PMCID: PMC3004519
- DOI: 10.1189/jlb.0610381
Store-operated Ca²+ signaling in dendritic cells occurs independently of STIM1
Abstract
SOCE via CRAC channels is a critical signaling event in immune cells. Recent studies have identified key proteins underlying this process; STIM is an ER Ca²+ sensor that interacts with Orai, an intrinsic, pore-forming protein of the CRAC channel. In heterologous expression systems, STIM1 regulates SOCE by interacting with Orai1, -2, and -3. In native tissues, however, the precise roles of STIM and Orai proteins are not well defined. Here, we have investigated the molecular components of SOCE signaling in mouse DCs. We show that DCs predominantly express STIM2 and only very low levels of STIM1 compared with T lymphocytes. Upon store depletion with Tg, STIM2 aggregates and interacts selectively with Orai2. In contrast, Tg fails to aggregate STIM1 or enhance STIM1-mediated interactions with Orai proteins. Consistent with this biochemical characterization, stimulation of DCs with the adhesion molecule ICAM-1 selectively recruits STIM2 and Orai2 to the IS. Together, these data demonstrate a novel, STIM2-dependent SOCE signaling pathway in DCs.
Figures
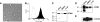
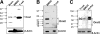


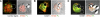
References
-
- Hsu S.f., O'Connell P. J., Klyachko V. A., Badminton M. N., Thomson A. W., Jackson M. B., Clapham D. E., Ahern G. P. (2001) Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J. Immunol. 166, 6126–6133 - PubMed
-
- Czerniecki B. J., Carter C., Rivoltini L., Koski G. K., Kim H. I., Weng D. E., Roros J. G., Hijazi Y. M., Xu S., Rosenberg S. A., Cohen P. A. (1997) Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J. Immunol. 159, 3823–3837 - PubMed
-
- Koski G. K., Schwartz G. N., Weng D. E., Czerniecki B. J., Carter C., Gress R. E., Cohen P. A. (1999) Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J. Immunol. 163, 82–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

