Cyclooxygenase-2 enhances antimicrobial peptide expression and killing of Staphylococcus aureus
- PMID: 20971925
- PMCID: PMC3025174
- DOI: 10.4049/jimmunol.1002009
Cyclooxygenase-2 enhances antimicrobial peptide expression and killing of Staphylococcus aureus
Abstract
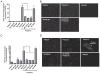
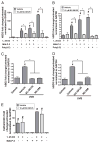
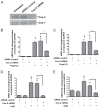
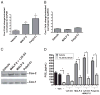
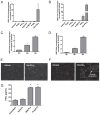
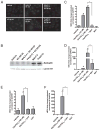
Antimicrobial peptides such as human β-defensins (hBDs) and cathelicidins are critical for protection against infection and can be induced by activation of TLRs, a pathway that also activates cyclooxygenase(Cox)-2 expression. We hypothesized that Cox-2 is induced by TLR activation and is necessary for optimal AMP production, and that inhibitors of Cox-2 may therefore inhibit antimicrobial action. Normal human keratinocytes (NHEKs) stimulated with a TLR2/6 ligand, macrophage-activating lipopeptide-2, or a TLR3 ligand, polyinosinic-polycytidylic acid, increased Cox-2 mRNA and protein and increased PGE(2), a product of Cox-2. Treatment with a Cox-2 selective inhibitor (SC-58125) or Cox-2 small interfering RNA attenuated hBD2 and hBD3 production in NHEKs when stimulated with macrophage-activating lipopeptide-2, polyinosinic-polycytidylic acid, or UVB (15 mJ/cm(2)), but it did not attenuate vitamin D3-induced cathelicidin. SC-58125 also inhibited TLR-dependent NF-κB activation. Conversely, treatment with Cox-derived prostanoids PGD(2) or 15-deoxy-Δ(12,14)-PGJ(2) induced hBD3 or hBD2 and hBD3, respectively. The functional significance of these observations was seen in NHEKs that showed reduced anti-staphylococcal activity when treated with a Cox-2 inhibitor. These findings demonstrate a critical role for Cox-2 in hBD production and suggest that the use of Cox-2 inhibitors may adversely influence the risk for bacterial infection.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating β-defensin production.Mol Cell Biol. 2014 Dec;34(24):4368-78. doi: 10.1128/MCB.00599-14. Epub 2014 Oct 13. Mol Cell Biol. 2014. PMID: 25312644 Free PMC article.
-
Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes.Infect Immun. 2003 Jul;71(7):3730-9. doi: 10.1128/IAI.71.7.3730-3739.2003. Infect Immun. 2003. PMID: 12819054 Free PMC article.
-
Pimecrolimus enhances TLR2/6-induced expression of antimicrobial peptides in keratinocytes.J Invest Dermatol. 2008 Nov;128(11):2646-2654. doi: 10.1038/jid.2008.135. Epub 2008 May 22. J Invest Dermatol. 2008. PMID: 18496569 Free PMC article.
-
Selective induction of antimicrobial peptides from keratinocytes by staphylococcal bacteria.Microb Pathog. 2013 Mar;56:35-9. doi: 10.1016/j.micpath.2012.11.005. Epub 2012 Nov 20. Microb Pathog. 2013. PMID: 23178253
-
Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells.Microbes Infect. 2006 Feb;8(2):380-9. doi: 10.1016/j.micinf.2005.07.006. Epub 2005 Sep 15. Microbes Infect. 2006. PMID: 16242370 Free PMC article.
Cited by
-
Regulation of inducible heme oxygenase and cyclooxygenase isozymes in a mouse model of spotted fever group rickettsiosis.Microb Pathog. 2012 Jul;53(1):28-36. doi: 10.1016/j.micpath.2012.03.010. Epub 2012 Apr 10. Microb Pathog. 2012. PMID: 22522044 Free PMC article.
-
3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88.PLoS One. 2011;6(8):e24008. doi: 10.1371/journal.pone.0024008. Epub 2011 Aug 25. PLoS One. 2011. PMID: 21901151 Free PMC article.
-
Comparison of Efficacy of Doxycycline and Isotretinoin on Cutaneous Human Beta-Defensin-1 and -2 Levels in Acne Vulgaris.Indian J Dermatol. 2018 Sep-Oct;63(5):380-385. doi: 10.4103/ijd.IJD_402_16. Indian J Dermatol. 2018. PMID: 30210158 Free PMC article.
-
Efficacy of celery (Apium graveolens L.) alcoholic extract against systemic methicillin-resistant Staphylococcus aureus infection in rat models.Vet World. 2022 Apr;15(4):898-905. doi: 10.14202/vetworld.2022.898-905. Epub 2022 Apr 12. Vet World. 2022. PMID: 35698524 Free PMC article.
-
An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating β-defensin production.Mol Cell Biol. 2014 Dec;34(24):4368-78. doi: 10.1128/MCB.00599-14. Epub 2014 Oct 13. Mol Cell Biol. 2014. PMID: 25312644 Free PMC article.
References
-
- Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. - PubMed
-
- Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002;30:185–194. - PubMed
-
- Pivarcsi A, Kemény L, Dobozy A. Innate immune functions of the keratinocytes: a review. Acta Microbiol Immunol Hung. 2004;51:303–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

