Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia
- PMID: 20971952
- PMCID: PMC3037741
- DOI: 10.1182/blood-2010-01-262808
Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia
Abstract
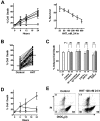
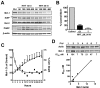
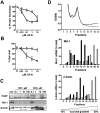
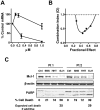
Homoharringtonine (HHT) is a plant alkaloid that inhibits the elongation phase of translation that is currently in clinical trials. Because the intrinsically short-lived antiapoptotic protein myeloid cell leukemia-1 (Mcl-1) has been reported to support the survival of chronic lymphocytic leukemia (CLL) cells, we hypothesized that inhibition of protein synthesis by HHT would decrease Mcl-1 expression and induce apoptosis in CLL. In primary CLL cells, HHT induced significant apoptosis independent of the prognostic characteristics of the patients. This was associated with inhibition of translation and decreased Mcl-1 levels in CLL cells. Mcl-1 reduction was evident as early as 2 hours and continued to decrease in the next 6-8 hours, whereas cell death started in 2 hours and continued to increase for 24 hours. Reduction of the Mcl-1 level was due to translation inhibition and proteasome degradation rather than to transcription inhibition or caspase cleavage. HHT and the transcription inhibitor SNS-032 induced synergistic cell killing. Although stromal cells induced Mcl-1 expression and protected CLL cells from the toxicity of fludarabine, this induction was reversed by HHT, which overcame stromal cell-mediated protection. Thus, these results provide a rationale for clinical development of HHT in CLL as single agent or in combinations.
Figures

References
-
- Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10(3):456–459. - PubMed
-
- Pepper C, Lin TT, Pratt G, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–3817. - PubMed
-
- Longo PG, Laurenti L, Gobessi S, et al. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

