Production of functional glucagon-secreting α-cells from human embryonic stem cells
- PMID: 20971966
- PMCID: PMC3012176
- DOI: 10.2337/db10-0573
Production of functional glucagon-secreting α-cells from human embryonic stem cells
Abstract
Objective: Differentiation of human embryonic stem (hES) cells to fully developed cell types holds great therapeutic promise. Despite significant progress, the conversion of hES cells to stable, fully differentiated endocrine cells that exhibit physiologically regulated hormone secretion has not yet been achieved. Here we describe an efficient differentiation protocol for the in vitro conversion of hES cells to functional glucagon-producing α- cells.
Research design and methods: Using a combination of small molecule screening and empirical testing, we developed a six-stage differentiation protocol for creating functional α-cells. An extensive in vitro and in vivo characterization of the differentiated cells was performed.
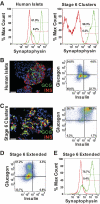
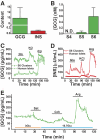
Results: A high rate of synaptophysin expression (>75%) and robust expression of glucagon and the α-cell transcription factor ARX was achieved. After a transient polyhormonal state in which cells coexpress glucagon and insulin, maturation in vitro or in vivo resulted in depletion of insulin and other β-cell markers with concomitant enrichment of α-cell markers. After transplantation, these cells secreted fully processed, biologically active glucagon in response to physiologic stimuli including prolonged fasting and amino acid challenge. Moreover, glucagon release from transplanted cells was sufficient to reduce demand for pancreatic glucagon, resulting in a significant decrease in pancreatic α-cell mass.
Conclusions: These results indicate that fully differentiated pancreatic endocrine cells can be created via stepwise differentiation of hES cells. These cells may serve as a useful screening tool for the identification of compounds that modulate glucagon secretion as well as those that promote the transdifferentiation of α-cells to β-cells.
Figures



References
-
- Alper J: Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol 2009;27:213–214 - PubMed
-
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE: Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 2005;23:1534–1541 - PubMed
-
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE: Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 - PubMed
-
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS: Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25:1940–1953 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

