Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice
- PMID: 20972332
- PMCID: PMC2964994
- DOI: 10.1172/JCI43873
Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice
Erratum in
- J Clin Invest. 2010 Dec 1;120(12):4583
Abstract
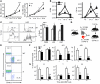
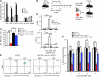
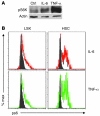
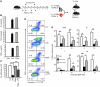
The mammalian target of rapamycin (mTOR) is a signaling molecule that senses environmental cues, such as nutrient status and oxygen supply, to regulate cell growth, proliferation, and other functions. Unchecked, sustained mTOR activity results in defects in HSC function. Inflammatory conditions, such as autoimmune disease, are often associated with defective hematopoiesis. Here, we investigated whether hyperactivation of mTOR in HSCs contributes to hematopoietic defects in autoimmunity and inflammation. We found that in mice deficient in Foxp3 (scurfy mice), a model of autoimmunity, the development of autoimmune disease correlated with progressive bone marrow loss and impaired regenerative capacity of HSCs in competitive bone marrow transplantation. Similarly, LPS-mediated inflammation in C57BL/6 mice led to massive bone marrow cell death and impaired HSC function. Importantly, treatment with rapamycin in both models corrected bone marrow hypocellularity and partially restored hematopoietic activity. In cultured mouse bone marrow cells, treatment with either of the inflammatory cytokines IL-6 or TNF-α was sufficient to activate mTOR, while preventing mTOR activation in vivo required simultaneous inhibition of CCL2, IL-6, and TNF-α. These data strongly suggest that mTOR activation in HSCs by inflammatory cytokines underlies defective hematopoiesis in autoimmune disease and inflammation.
Figures



Comment in
-
Pharmacologic eigenvalues: beating the rap on bone marrow failure.J Clin Invest. 2010 Nov;120(11):3813-5. doi: 10.1172/JCI45060. Epub 2010 Oct 25. J Clin Invest. 2010. PMID: 20972329 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

