Support for a synaptic chain model of neuronal sequence generation
- PMID: 20972420
- PMCID: PMC2998755
- DOI: 10.1038/nature09514
Support for a synaptic chain model of neuronal sequence generation
Abstract
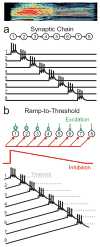
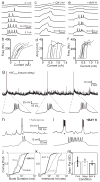
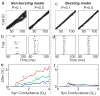
In songbirds, the remarkable temporal precision of song is generated by a sparse sequence of bursts in the premotor nucleus HVC. To distinguish between two possible classes of models of neural sequence generation, we carried out intracellular recordings of HVC neurons in singing zebra finches (Taeniopygia guttata). We found that the subthreshold membrane potential is characterized by a large, rapid depolarization 5-10 ms before burst onset, consistent with a synaptically connected chain of neurons in HVC. We found no evidence for the slow membrane potential modulation predicted by models in which burst timing is controlled by subthreshold dynamics. Furthermore, bursts ride on an underlying depolarization of ∼10-ms duration, probably the result of a regenerative calcium spike within HVC neurons that could facilitate the propagation of activity through a chain network with high temporal precision. Our results provide insight into the fundamental mechanisms by which neural circuits can generate complex sequential behaviours.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Lashley K. In: Cerebral mechanisms in behavior. Jeffress L, editor. Wiley; 1951.
-
- Shima K, Isoda M, Mushiake H, Tanji J. Categorization of behavioural sequences in the prefrontal cortex. Nature. 2007;445:315–318. - PubMed
-
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

