Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells
- PMID: 20972423
- PMCID: PMC2972406
- DOI: 10.1038/ncb2108
Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells
Abstract
Endothelial cells establish an instructive vascular niche that reconstitutes haematopoietic stem and progenitor cells (HSPCs) through release of specific paracrine growth factors, known as angiocrine factors. However, the mechanism by which endothelial cells balance the rate of proliferation and lineage-specific differentiation of HSPCs is unknown. Here, we demonstrate that Akt activation in endothelial cells, through recruitment of mTOR, but not the FoxO pathway, upregulates specific angiocrine factors that support expansion of CD34(-)Flt3(-) KLS HSPCs with long-term haematopoietic stem cell (LT-HSC) repopulation capacity. Conversely, co-activation of Akt-stimulated endothelial cells with p42/44 MAPK shifts the balance towards maintenance and differentiation of the HSPCs. Selective activation of Akt1 in the endothelial cells of adult mice increased the number of colony forming units in the spleen and CD34(-)Flt3(-) KLS HSPCs with LT-HSC activity in the bone marrow, accelerating haematopoietic recovery. Therefore, the activation state of endothelial cells modulates reconstitution of HSPCs through the modulation of angiocrine factors, with Akt-mTOR-activated endothelial cells supporting the self-renewal of LT-HSCs and expansion of HSPCs, whereas MAPK co-activation favours maintenance and lineage-specific differentiation of HSPCs.
Conflict of interest statement
Figures
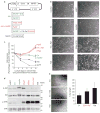

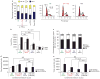

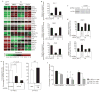


Comment in
-
HSPCs in the balance: The vascular niche.Cell Stem Cell. 2010 Dec 3;7(6):645-6. doi: 10.1016/j.stem.2010.11.021. Cell Stem Cell. 2010. PMID: 21112557 No abstract available.
Similar articles
-
Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells.Cell Stem Cell. 2010 Mar 5;6(3):251-64. doi: 10.1016/j.stem.2010.02.001. Cell Stem Cell. 2010. PMID: 20207228 Free PMC article.
-
Conversion of adult endothelium to immunocompetent haematopoietic stem cells.Nature. 2017 May 25;545(7655):439-445. doi: 10.1038/nature22326. Epub 2017 May 17. Nature. 2017. PMID: 28514438 Free PMC article.
-
Endothelial mTOR maintains hematopoiesis during aging.J Exp Med. 2020 Jun 1;217(6):e20191212. doi: 10.1084/jem.20191212. J Exp Med. 2020. PMID: 32289154 Free PMC article.
-
The Akt-mTOR network at the interface of hematopoietic stem cell homeostasis.Exp Hematol. 2021 Nov;103:15-23. doi: 10.1016/j.exphem.2021.08.009. Epub 2021 Aug 28. Exp Hematol. 2021. PMID: 34464661 Review.
-
Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow.Exp Cell Res. 2014 Dec 10;329(2):239-47. doi: 10.1016/j.yexcr.2014.09.016. Epub 2014 Sep 22. Exp Cell Res. 2014. PMID: 25246130 Review.
Cited by
-
E-selectin mediates stem cell adhesion and formation of blood vessels in a murine model of infantile hemangioma.Am J Pathol. 2012 Dec;181(6):2239-47. doi: 10.1016/j.ajpath.2012.08.030. Epub 2012 Oct 4. Am J Pathol. 2012. PMID: 23041613 Free PMC article.
-
In vitro conversion of adult murine endothelial cells to hematopoietic stem cells.Nat Protoc. 2018 Dec;13(12):2758-2780. doi: 10.1038/s41596-018-0060-3. Nat Protoc. 2018. PMID: 30429596 Free PMC article.
-
CELLPEDIA: a repository for human cell information for cell studies and differentiation analyses.Database (Oxford). 2011 Oct 29;2011:bar046. doi: 10.1093/database/bar046. Print 2011. Database (Oxford). 2011. PMID: 22039163 Free PMC article.
-
Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway.EMBO Rep. 2011 May;12(5):470-6. doi: 10.1038/embor.2011.39. Epub 2011 Apr 1. EMBO Rep. 2011. PMID: 21460795 Free PMC article.
-
The secretome of endothelial progenitor cells promotes brain endothelial cell activity through PI3-kinase and MAP-kinase.PLoS One. 2014 Apr 22;9(4):e95731. doi: 10.1371/journal.pone.0095731. eCollection 2014. PLoS One. 2014. PMID: 24755675 Free PMC article.
References
-
- Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. - PubMed
-
- Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
-
- Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. - PubMed
-
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. - PubMed
-
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

