Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses
- PMID: 20972432
- PMCID: PMC2982944
- DOI: 10.1038/ni.1953
Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses
Abstract
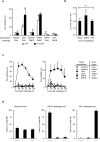
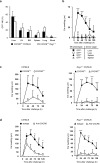
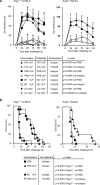
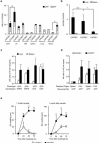
Hepatic natural killer (NK) cells mediate antigen-specific contact hypersensitivity (CHS) in mice deficient in T cells and B cells. We report here that hepatic NK cells, but not splenic or naive NK cells, also developed specific memory of vaccines containing antigens from influenza, vesicular stomatitis virus (VSV) or human immunodeficiency virus type 1 (HIV-1). Adoptive transfer of virus-sensitized NK cells into naive recipient mice enhanced the survival of the mice after lethal challenge with the sensitizing virus but not after lethal challenge with a different virus. NK cell memory of haptens and viruses depended on CXCR6, a chemokine receptor on hepatic NK cells that was required for the persistence of memory NK cells but not for antigen recognition. Thus, hepatic NK cells can develop adaptive immunity to structurally diverse antigens, an activity that requires NK cell-expressed CXCR6.
Figures


Comment in
-
More things in heaven and earth: defining innate and adaptive immunity.Nat Immunol. 2010 Dec;11(12):1080-2. doi: 10.1038/ni1210-1080. Nat Immunol. 2010. PMID: 21079631 Review.
-
Making memories.Nat Rev Immunol. 2010 Dec;10(12):811. doi: 10.1038/nri2895. Nat Rev Immunol. 2010. PMID: 21155196 No abstract available.
References
-
- Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–S55. - PubMed
-
- Crowle AJ. Delayed hypersensitivity in mice; its detection by skin tests and its passive transfer. Science. 1959;130:159–160. - PubMed
-
- Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–312. - PubMed
-
- Phanuphak P, Moorhead JW, Claman HN. Tolerance and contact sensitivity to DNFB in mice. II. Specific in vitro stimulation with a hapten, 2,4-dinitrobenzene sulfonic acid (DNBSO3Na) J Immunol. 1974;112:849–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

