Molecular organization of the COG vesicle tethering complex
- PMID: 20972446
- PMCID: PMC3113405
- DOI: 10.1038/nsmb.1917
Molecular organization of the COG vesicle tethering complex
Abstract
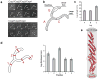
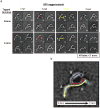
Multisubunit tethering complexes of the CATCHR (complexes associated with tethering containing helical rods) family are proposed to mediate the initial contact between an intracellular trafficking vesicle and its membrane target. To begin elucidating the molecular architecture of one well-studied example, the conserved oligomeric Golgi (COG) complex, we reconstituted its essential subunits (Cog1, Cog2, Cog3 and Cog4) and used single-particle electron microscopy to reveal a y-shaped structure with three flexible, highly extended legs. Labeling experiments established that the N termini of all four subunits interact along the proximal segment of one leg, whereas three of the four C termini are located at the tips of the legs. Our results suggest that the central region of the Cog1-Cog2-Cog3-Cog4 complex, as well as the distal regions of at least two legs, all participate in interactions with other components of the intracellular trafficking machinery.
Figures



References
-
- Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. - PubMed
-
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. - PubMed
-
- Yu I, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Ann Rev Cell Dev Biol. 2010;26 in press. - PubMed
-
- Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16:113–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

