Analysis of epoxyeicosatrienoic acids by chiral liquid chromatography/electron capture atmospheric pressure chemical ionization mass spectrometry using [13C]-analog internal standards
- PMID: 20972997
- PMCID: PMC3348553
- DOI: 10.1002/rcm.4760
Analysis of epoxyeicosatrienoic acids by chiral liquid chromatography/electron capture atmospheric pressure chemical ionization mass spectrometry using [13C]-analog internal standards
Abstract
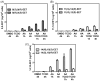
The metabolism of arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs) is thought to be mediated primarily by the cytochromes P450 (P450s) from the 2 family (2C9, 2C19, 2D6, and 2J2). In contrast, P450s of the 4 family are primarily involved in omega oxidation of AA (4A11 and 4A22). The ability to determine enantioselective formation of the regioisomeric EETs is important in order to establish their potential biological activities and to asses which P450 isoforms are involved in their formation. It has been extremely difficult to analyze individual EET enantiomers in biological fluids because they are present in only trace amounts and they are extremely difficult to separate from each other. In addition, the deuterium-labeled internal standards that are commonly used for stable isotope dilution liquid chromatography/mass spectrometry (LC/MS) analyses have different LC retention times when compared with the corresponding protium forms. Therefore, quantification by LC/MS-based methodology can be compromised by differential suppression of ionization of the closely eluting isomers. We report the preparation of [(13)C(20)]-EET analog internal standards and the use of a validated high-sensitivity chiral LC/electron capture atmospheric pressure chemical ionization (ECAPCI)-MS method for the trace analysis of endogenous EETs as their pentafluorobenzyl (PFB) ester derivatives. The assay was then used to show the exquisite enantioselectivity of P4502C19-, P4502D6-, P4501A1-, and P4501B1-mediated conversion of AA into EETs and to quantify the enantioselective formation of EETs produced by AA metabolism in a mouse epithelial hepatoma (Hepa) cell line.
2010 John Wiley & Sons, Ltd.
Figures









References
-
- Guengerich FP. Drug Metab Rev. 2004;36:159. - PubMed
-
- Capdevila JH, Falck JR, Imig JD. Kidney Int. 2007;72:683. - PubMed
-
- Guengerich FP. Chem Res Toxicol. 2008;21:70. - PubMed
-
- Bylund J, Kunz T, Valmsen K, Oliw EH. J Pharmacol Exp Ther. 1998;284:51. - PubMed
-
- Hsu MH, Savas U, Griffin KJ, Johnson EF. Drug Metab Rev. 2007;39:515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
