Classical conditioning of autonomic fear responses is independent of contingency awareness
- PMID: 20973611
- PMCID: PMC6510249
- DOI: 10.1037/a0020263
Classical conditioning of autonomic fear responses is independent of contingency awareness
Abstract
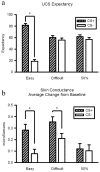
The role of contingency awareness in classical conditioning experiments using human subjects is currently under debate. This study took a novel approach to manipulating contingency awareness in a differential Pavlovian conditioning paradigm. Complex sine wave gratings were used as visual conditional stimuli (CS). By manipulating the fundamental spatial frequency of the displays, we were able to construct pairs of stimuli that varied in discriminability. One group of subjects was given an "easy" discrimination, and another was exposed to a "difficult" CS+ and CS-. A 3rd group was exposed to a stimulus that was paired with the unconditional stimulus (UCS) 50% of the time and served as a control. Skin conductance response (SCR) and continuous UCS expectancy data were measured concurrently throughout the experiment. Differential UCS expectancy was found only in the easy discrimination group. Differential SCRs were found in the easy discrimination group as well as in the difficult discrimination group, but not in the 50% contingency control. The difficult discrimination group did not exhibit differential UCS expectancy but did show clear differential SCR. These observations support a dual process interpretation of classical conditioning whereby conditioning on an implicit level can occur without explicit knowledge about the contingencies. The role of contingency awareness in classical conditioning experiments using human subjects is currently under debate. This study took a novel approach to manipulating contingency awareness in a differential Pavlovian conditioning paradigm. Complex sine wave gratings were used as visual conditional stimuli (CS). By manipulating the fundamental spatial frequency of the displays, we were able to construct pairs of stimuli that varied in discriminability. One group of subjects was given an "easy" discrimination, and another was exposed to a "difficult" CS+ and CS-. A 3rd group was exposed to a stimulus that was paired with the unconditional stimulus (UCS) 50% of the time and served as a control. Skin conductance response (SCR) and continuous UCS expectancy data were measured concurrently throughout the experiment. Differential UCS expectancy was found only in the easy discrimination group. Differential SCRs were found in the easy discrimination group as well as in the difficult discrimination group, but not in the 50% contingency control. The difficult discrimination group did not exhibit differential UCS expectancy but did show clear differential SCR. These observations support a dual process interpretation of classical conditioning whereby conditioning on an implicit level can occur without explicit knowledge about the contingencies.
2010 APA, all rights reserved
Figures

Similar articles
-
Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies.Neuroimage. 2006 Aug 15;32(2):761-70. doi: 10.1016/j.neuroimage.2006.03.038. Epub 2006 May 2. Neuroimage. 2006. PMID: 16651009
-
Dissociation between implicit and explicit responses in postconditioning UCS revaluation after fear conditioning in humans.Behav Neurosci. 2013 Jun;127(3):357-68. doi: 10.1037/a0032742. Behav Neurosci. 2013. PMID: 23731073 Free PMC article. Clinical Trial.
-
Conditioning with masked stimuli affects the timecourse of skin conductance responses.Behav Neurosci. 2010 Aug;124(4):478-89. doi: 10.1037/a0019927. Behav Neurosci. 2010. PMID: 20695647 Free PMC article.
-
A systematic review and meta-analysis of the evidence for unaware fear conditioning.Neurosci Biobehav Rev. 2020 Jan;108:254-268. doi: 10.1016/j.neubiorev.2019.11.012. Epub 2019 Nov 17. Neurosci Biobehav Rev. 2020. PMID: 31747553
-
UCS revaluation and conditioning models of acquired fears.Behav Res Ther. 1989;27(5):521-8. doi: 10.1016/0005-7967(89)90086-7. Behav Res Ther. 1989. PMID: 2684133 Review.
Cited by
-
Awareness and differential eyeblink conditioning: effects of manipulating auditory CS frequencies.Learn Mem. 2020 Jan 16;27(2):78-82. doi: 10.1101/lm.050146.119. Print 2020 Feb. Learn Mem. 2020. PMID: 31949039 Free PMC article.
-
Eye Movements Index Implicit Memory Expression in Fear Conditioning.PLoS One. 2015 Nov 12;10(11):e0141949. doi: 10.1371/journal.pone.0141949. eCollection 2015. PLoS One. 2015. PMID: 26562298 Free PMC article.
-
The Role of Implicit Memory in the Development and Recovery from Trauma-Related Disorders.NeuroSci. 2022 Jan 18;3(1):63-88. doi: 10.3390/neurosci3010005. eCollection 2022 Mar. NeuroSci. 2022. PMID: 39484673 Free PMC article.
-
Implicit but not explicit extinction to threat-conditioned stimulus prevents spontaneous recovery of threat-potentiated startle responses in humans.Brain Behav. 2019 Jan;9(1):e01157. doi: 10.1002/brb3.1157. Epub 2018 Dec 4. Brain Behav. 2019. PMID: 30516021 Free PMC article.
-
Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety.Front Behav Neurosci. 2014 Feb 28;8:32. doi: 10.3389/fnbeh.2014.00032. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24616672 Free PMC article.
References
-
- Baer P, Fuhrer M. Cognitive processes during differential trace and delayed conditioning of the GSR. Journal of Experimental Psychology. 1968;78:81–88. - PubMed
-
- Baeyens F, Eelen P, Van den Bergh O. Contingency awareness in evaluative conditioning: A case for unaware affective-evaluative learning. Cognition & Emotion. 1990;4:3–18.
-
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995 Aug 25;269:1115–1118. - PubMed
-
- Cheng D, Knight D, Smith C, Helmstetter F. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. - PubMed

