Succinic semialdehyde dehydrogenase: biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance
- PMID: 20973619
- PMCID: PMC3125545
- DOI: 10.1089/ars.2010.3470
Succinic semialdehyde dehydrogenase: biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance
Abstract
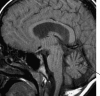
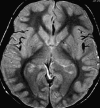
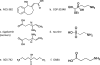
Succinic semialdehyde dehydrogenase (SSADH; aldehyde dehydrogenase 5a1, ALDH5A1; E.C. 1.2.1.24; OMIM 610045, 271980) deficiency is a rare heritable disorder that disrupts the metabolism of the inhibitory neurotransmitter 4-aminobutyric acid (GABA). Identified in conjunction with increased urinary excretion of the GABA analog gamma-hydroxybutyric acid (GHB), numerous patients have been identified worldwide and the autosomal-recessive disorder has been modeled in mice. The phenotype is one of nonprogressive neurological dysfunction in which seizures may be prominently displayed. The murine model is a reasonable phenocopy of the human disorder, yet the severity of the seizure disorder in the mouse exceeds that observed in SSADH-deficient patients. Abnormalities in GABAergic and GHBergic neurotransmission, documented in patients and mice, form a component of disease pathophysiology, although numerous other disturbances (metabolite accumulations, myelin abnormalities, oxidant stress, neurosteroid depletion, altered bioenergetics, etc.) are also likely to be involved in developing the disease phenotype. Most recently, the demonstration of a redox control system in the SSADH protein active site has provided new insights into the regulation of SSADH by the cellular oxidation/reduction potential. The current review summarizes some 30 years of research on this protein and disease, addressing pathological mechanisms in human and mouse at the protein, metabolic, molecular, and whole-animal level.
Figures
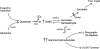









References
-
- Ahn JW. Kim YG. Kim KJ. Crystal structure of non-redox regulated SSADH from Escherichia coli. Biochem Biophys Res Commun. 2010;392:106–111. - PubMed
-
- Akaboshi S. Hogema BM. Novelletto A. Malaspina P. Salomons GS. Maropoulos GD. Jakobs C. Grompe M. Gibson KM. Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease-causing mutations in patients with SSADH deficiency. Hum Mutat. 2003;22:442–450. - PubMed
-
- Albers RW. Koval GJ. Succinic semialdehyde dehydrogenase: purification and properties of the enzyme from monkey brain. Biochim Biophys Acta. 1961;52:29–35. - PubMed
-
- Albers RW. Salvador RA. Succinic semialdehyde oxidation by a soluble dehydrogenase from brain. Science. 1958;128:359–360. - PubMed
-
- Al-Essa MA. Bakheet SM. Patay ZJ. Powe JE. Ozand PT. Clinical, fluorine-18 labeled 2-fluoro-2-deoxyglucose positron emission tomography (FDG PET), MRI of the brain and biochemical observations in a patient with 4-hydroxybutyric aciduria; a progressive neurometabolic disease. Brain Dev. 2000;22:127–131. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

