Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro
- PMID: 20973749
- PMCID: PMC3043983
- DOI: 10.1089/ten.TEA.2010.0377
Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro
Abstract
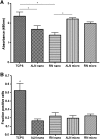
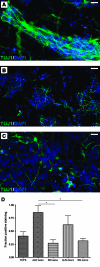
The potential of human embryonic stem (ES) cells as experimental therapies for neuronal replacement has recently received considerable attention. In view of the organization of the mature nervous system into distinct neural circuits, key challenges of such therapies are the directed differentiation of human ES cell-derived neural precursors (NPs) into specific neuronal types and the directional growth of axons along specified trajectories. In the present study, we cultured human NPs derived from the NIH-approved ES line BGO1 on polycaprolactone fiber matrices of different diameter (i.e., nanofibers and microfibers) and orientation (i.e., aligned and random); fibers were coated with poly-L-ornithine/laminin to mimic the extracellular matrix and support the adhesion, viability, and differentiation of NPs. On aligned fibrous meshes, human NPs adopt polarized cell morphology with processes extending along the axis of the fiber, whereas NPs on plain tissue culture surfaces or random fiber substrates form nonpolarized neurite networks. Under differentiation conditions, human NPs cultured on aligned fibrous substrates show a higher rate of neuronal differentiation than other matrices; 62% and 86% of NPs become TUJ1 (+) early neurons on aligned micro- and nanofibers, respectively, whereas only 32% and 27% of NPs acquire the same fate on random micro- and nanofibers. Metabolic cell activity/viability studies reveal that fiber alignment and diameter also have an effect on NP viability, but only in the presence of mitogens. Our findings demonstrate that fibrous substrates serve as an artificial extracellular matrix and provide a microenviroment that influences key aspects of the neuronal differentiation of ES-derived NPs.
Figures



Similar articles
-
The effects of electrospun TSF nanofiber diameter and alignment on neuronal differentiation of human embryonic stem cells.J Biomed Mater Res A. 2012 Mar;100(3):632-45. doi: 10.1002/jbm.a.33291. Epub 2011 Dec 30. J Biomed Mater Res A. 2012. PMID: 22213384
-
Influence of oriented nanofibrous PCL scaffolds on quantitative gene expression during neural differentiation of mouse embryonic stem cells.J Biomed Mater Res A. 2016 Jan;104(1):155-64. doi: 10.1002/jbm.a.35551. Epub 2015 Aug 25. J Biomed Mater Res A. 2016. PMID: 26255987
-
Neuronal vs. glial fate of embryonic stem cell-derived neural progenitors (ES-NPs) is determined by FGF2/EGF during proliferation.J Mol Neurosci. 2010 Sep;42(1):17-27. doi: 10.1007/s12031-010-9335-z. Epub 2010 Feb 13. J Mol Neurosci. 2010. PMID: 20155332
-
Programming embryonic stem cells to neuronal subtypes.Curr Opin Neurobiol. 2011 Feb;21(1):43-51. doi: 10.1016/j.conb.2010.09.012. Epub 2010 Oct 20. Curr Opin Neurobiol. 2011. PMID: 20970319 Free PMC article. Review.
-
Conductive Biomaterials as Substrates for Neural Stem Cells Differentiation towards Neuronal Lineage Cells.Macromol Biosci. 2021 Jan;21(1):e2000123. doi: 10.1002/mabi.202000123. Epub 2020 Oct 4. Macromol Biosci. 2021. PMID: 33015992 Review.
Cited by
-
Electrospun nanofibers as versatile interfaces for efficient gene delivery.J Biol Eng. 2014 Dec 9;8:30. doi: 10.1186/1754-1611-8-30. eCollection 2014. J Biol Eng. 2014. PMID: 25926887 Free PMC article. Review.
-
Biological Effects of Culture Substrates on Human Pluripotent Stem Cells.Stem Cells Int. 2016;2016:5380560. doi: 10.1155/2016/5380560. Epub 2016 Aug 30. Stem Cells Int. 2016. PMID: 27656216 Free PMC article. Review.
-
Nanotopography-guided tissue engineering and regenerative medicine.Adv Drug Deliv Rev. 2013 Apr;65(4):536-58. doi: 10.1016/j.addr.2012.07.014. Epub 2012 Aug 18. Adv Drug Deliv Rev. 2013. PMID: 22921841 Free PMC article. Review.
-
Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration.Biomater Sci. 2018 May 29;6(6):1299-1311. doi: 10.1039/c8bm00260f. Biomater Sci. 2018. PMID: 29725688 Free PMC article. Review.
-
Accelerated neural differentiation of mouse embryonic stem cells on aligned GYIGSR-functionalized nanofibers.Acta Biomater. 2018 Jul 15;75:129-139. doi: 10.1016/j.actbio.2018.05.052. Epub 2018 Jun 5. Acta Biomater. 2018. PMID: 29879551 Free PMC article.
References
-
- Tabar V. Panagiotakos G. Greenberg E.D. Chan B.K. Sadelain M. Gutin P.H. Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23:601. - PubMed
-
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

