Roles of affinity and lipophilicity in the slow kinetics of prostanoid receptor antagonists on isolated smooth muscle preparations
- PMID: 20973775
- PMCID: PMC3042197
- DOI: 10.1111/j.1476-5381.2010.01087.x
Roles of affinity and lipophilicity in the slow kinetics of prostanoid receptor antagonists on isolated smooth muscle preparations
Abstract
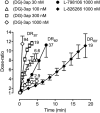
Background and purpose: The highly lipophilic acyl-sulphonamides L-798106 and L-826266 showed surprisingly slow antagonism of the prostanoid EP₃ receptor system in guinea-pig aorta. Roles of affinity and lipophilicity in the onset kinetics of these and other prostanoid ligands were investigated.
Experimental approach: Antagonist selectivity was assessed using a panel of human recombinant prostanoid receptor-fluorimetric imaging plate reader assays. Potencies/affinities and onset half-times of agonists and antagonists were obtained on guinea-pig-isolated aorta and vas deferens. n-Octanol-water partition coefficients were predicted.
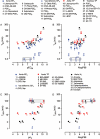
Key results: L-798106, L-826266 and the less lipophilic congener (DG)-3ap appear to behave as selective, competitive-reversible EP₃ antagonists. For ligands of low to moderate lipophilicity, potency increments for EP₃ and TP (thromboxane-like) agonism on guinea-pig aorta (above pEC₅₀ of 8.0) were associated with progressively longer onset half-times; similar trends were found for TP and histamine H₁ antagonism above a pA₂ limit of 8.0. In contrast, L-798106 (EP₃), L-826266 (EP₃, TP) and the lipophilic H₁ antagonists astemizole and terfenadine exhibited very slow onset rates despite their moderate affinities; (DG)-3ap (EP₃) had a faster onset. Agonism and antagonism on the vas deferens EP₃ system were overall much faster, although trends were similar.
Conclusions and implications: High affinity and high liphophilicity may contribute to the slow onsets of prostanoid ligands in some isolated smooth muscle preparations. Both relationships are explicable by tissue disposition under the limited diffusion model. EP₃ antagonists used as research tools should have moderate lipophilicity. The influence of lipophilicity on the potential clinical use of EP₃ antagonists is discussed.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
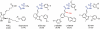


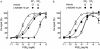
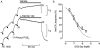

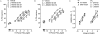
References
-
- Anderson GP. Formoterol: pharmacology, molecular basis of agonism, and mechanism of long duration of a highly potent and selective β2-adrenoceptor agonist bronchodilator. Life Sci. 1993;52:2145–2160. - PubMed
-
- Anderson GP, Lindén A, Rabe KF. Why are long-acting beta-adrenoceptor agonists long-acting? Eur Respir J. 1994;7:569–578. - PubMed
-
- Austin RP, Barton P, Bonnert RV, Brown RC, Cage PA, Cheshire DR, et al. QSAR and the rational design of long-acting dual D2-receptor/β2-adrenoceptor agonists. J Med Chem. 2003;46:3210–3220. - PubMed
-
- Avdeef A, Box KJ, Takács-Kovák K. pH-metric logP. 6. Effects of sodium, potassium and N-CH3-D-glucamine on the octanol-water partitioning of prostaglandins E1 and E2. J Pharm Sci. 1995;84:523–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

