CRF₂ mediates the increased noradrenergic activity in the hypothalamic paraventricular nucleus and the negative state of morphine withdrawal in rats
- PMID: 20973778
- PMCID: PMC3042196
- DOI: 10.1111/j.1476-5381.2010.01090.x
CRF₂ mediates the increased noradrenergic activity in the hypothalamic paraventricular nucleus and the negative state of morphine withdrawal in rats
Abstract
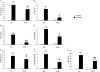
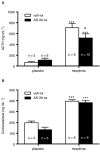
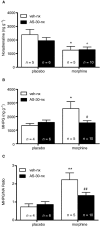
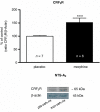
Background and purpose: Recent evidence suggests that corticotropin-releasing factor (CRF) receptor signalling is involved in modulating the negative symptoms of opiate withdrawal. In this study, a series of experiments were performed to further characterize the role of CRF-type 2 receptor (CRF₂) signalling in opiate withdrawal-induced physical signs of dependence, hypothalamus-pituitary-adrenal (HPA) axis activation, enhanced noradrenaline (NA) turnover in the hypothalamic paraventricular nucleus (PVN) and tyrosine hydroxylase (TH) phosphorylation (activation), as well as CRF₂ expression in the nucleus of the solitary tract-A₂ noradrenergic cell group (NTS-A₂).
Experimental approach: The contribution of CRF₂ signalling in opiate withdrawal was assessed by i.c.v. infusion of the selective CRF₂ antagonist, antisauvagine-30 (AS-30). Rats were implanted with two morphine (or placebo) pellets. Six days later, rats were pretreated with AS-30 or saline 10 min before naloxone and the physical signs of abstinence, the HPA axis activity, NA turnover, TH activation and CRF₂ expression were measured using immunoblotting, RIA, HPLC and immunohistochemistry.
Key results: Rats pretreated with AS-30 showed decreased levels of somatic signs of naloxone-induced opiate withdrawal, but the corticosterone response was not modified. AS-30 attenuated the increased production of the NA metabolite, 3-methoxy-4-hydroxyphenylglycol, as well as the enhanced NA turnover observed in morphine-withdrawn rats. Finally, AS-30 antagonized the TH phosphorylation at Serine40 induced by morphine withdrawal.
Conclusions and implications: These results suggest that physical signs of opiate withdrawal, TH activation and stimulation of noradrenergic pathways innervating the PVN are modulated by CRF₂ signalling. Furthermore, they indicate a marginal role for the HPA axis in CRF₂-mediation of opiate withdrawal.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

Similar articles
-
Glucocorticoid receptors participate in the opiate withdrawal-induced stimulation of rats NTS noradrenergic activity and in the somatic signs of morphine withdrawal.Br J Pharmacol. 2012 Aug;166(7):2136-47. doi: 10.1111/j.1476-5381.2012.01918.x. Br J Pharmacol. 2012. PMID: 22364199 Free PMC article.
-
Spironolactone decreases the somatic signs of opiate withdrawal by blocking the mineralocorticoid receptors (MR).Toxicology. 2014 Dec 4;326:36-43. doi: 10.1016/j.tox.2014.10.002. Epub 2014 Oct 11. Toxicology. 2014. PMID: 25308750
-
Morphine withdrawal-induced c-fos expression in the hypothalamic paraventricular nucleus is dependent on the activation of catecholaminergic neurones.J Neurochem. 2002 Oct;83(1):132-40. doi: 10.1046/j.1471-4159.2002.01123.x. J Neurochem. 2002. PMID: 12358736
-
Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms.Ann N Y Acad Sci. 2008 Dec;1148:428-38. doi: 10.1196/annals.1410.032. Ann N Y Acad Sci. 2008. PMID: 19120138 Review.
-
Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female.Brain Res. 2010 Dec 10;1364:153-63. doi: 10.1016/j.brainres.2010.08.036. Epub 2010 Aug 18. Brain Res. 2010. PMID: 20727865 Review.
Cited by
-
Hypothalamic orexin--a neurons are involved in the response of the brain stress system to morphine withdrawal.PLoS One. 2012;7(5):e36871. doi: 10.1371/journal.pone.0036871. Epub 2012 May 9. PLoS One. 2012. PMID: 22590628 Free PMC article.
-
Glucocorticoid receptors participate in the opiate withdrawal-induced stimulation of rats NTS noradrenergic activity and in the somatic signs of morphine withdrawal.Br J Pharmacol. 2012 Aug;166(7):2136-47. doi: 10.1111/j.1476-5381.2012.01918.x. Br J Pharmacol. 2012. PMID: 22364199 Free PMC article.
-
Involvement of noradrenergic transmission in the PVN on CREB activation, TORC1 levels, and pituitary-adrenal axis activity during morphine withdrawal.PLoS One. 2012;7(2):e31119. doi: 10.1371/journal.pone.0031119. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355339 Free PMC article.
-
Region-specific neuroadaptations of CRF1 and CRF2 expression following heroin exposure in female rats.Pharmacol Biochem Behav. 2025 Feb;247:173931. doi: 10.1016/j.pbb.2024.173931. Epub 2024 Dec 1. Pharmacol Biochem Behav. 2025. PMID: 39626795
-
Morphine administration modulates expression of Argonaute 2 and dopamine-related transcription factors involved in midbrain dopaminergic neurons function.Br J Pharmacol. 2013 Apr;168(8):1889-901. doi: 10.1111/bph.12083. Br J Pharmacol. 2013. PMID: 23215787 Free PMC article.
References
-
- Bobrovskaya L, Gilligan C, Bolster EK, Flaherty JJ, Dickson PW, Dunkley PR. Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem. 2007;100:479–489. - PubMed
-
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. - PubMed
-
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

