New cancer targets emerging from studies of the Von Hippel-Lindau tumor suppressor protein
- PMID: 20973793
- PMCID: PMC4784475
- DOI: 10.1111/j.1749-6632.2010.05781.x
New cancer targets emerging from studies of the Von Hippel-Lindau tumor suppressor protein
Abstract
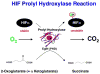
Inactivation of the von Hippel-Lindau tumor suppressor protein (pVHL) causes the most common form of kidney cancer. pVHL is part of a complex that polyubiquitinates the alpha subunit of the heterodimeric transcription factor HIF. In the presence of oxygen, HIF1α is prolyl hydroxylated by EglN1 (also called PHD2); this modification recruits pVHL, which then targets HIF1α for proteasomal degradation. In hypoxic or pVHL-defective cells, HIF1α accumulates, binds to HIF1β, and transcriptionally activates genes such as VEGF. VEGF inhibitors and mTOR inhibitors, which indirectly affect HIF, are now approved for the treatment of kidney cancer. EglN1 is a 2-oxoglutarate-dependent dioxygenase; such enzymes can be inhibited with drug-like small molecules and EglN1 inhibitors are currently being tested for the treatment of anemia. EglN2 (PHD1) and EglN3 (PHD3), which are EglN1 paralogs, appear to play HIF-independent roles in cell proliferation and apoptosis, respectively, and are garnering interest as potential cancer targets. A number of JmjC-containing proteins, including RBP2 and PLU-1, are 2-oxoglutarate-dependent dioxygenases that demethylate histones. Preclinical data suggest that inhibition of RBP2 or PLU-1 would suppress tumor growth.
© 2010 New York Academy of Sciences.
Figures

References
-
- Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. - PubMed
-
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
-
- Hirsila M, et al. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–80. - PubMed
-
- McNeill LA, et al. The use of dioxygen by HIF prolyl hydroxylase (PHD1) Bioorg Med Chem Lett. 2002;12:1547–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

