PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas
- PMID: 20973932
- PMCID: PMC3623294
- DOI: 10.1111/j.1755-148X.2010.00763.x
PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas
Abstract
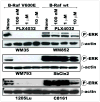
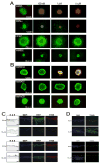
Targeted intervention of the B-Raf V600E gene product that is prominent in melanoma has been met with modest success. Here, we characterize the pharmacological properties of PLX4032, a next-generation inhibitor with exquisite specificity against the V600E oncogene and striking anti-melanoma activity. PLX4032 induces potent cell cycle arrest, inhibits proliferation, and initiates apoptosis exclusively in V600E-positive cells in a variety of in vitro experimental systems; follow-up xenograft studies demonstrate extreme selectivity and efficacy against melanoma tumors bearing the V600E oncoproduct. The collective data support further exploration of PLX4032 as a candidate drug for patients with metastatic melanoma; accordingly, validation of PLX4032 as a therapeutic tool for patients with melanoma is now underway in advanced human (Phase III) clinical trials.
Figures


References
-
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Dummer RRC, Chapman PB, Sosman JA, Middleton M, Bastholt L, Kemsley K, Cantarini MV, Morris C, Kirkwood JM. AZD6244 (ARRY-a428896) versus temozolomide (TMZ) in patients with advanced melanoma: An open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26:Abstract 9033.
-
- Flaherty KPI, Sosman J, Kim K, Ribas A, McArthur G, Lee RJ, Grippo JF, Nolop K, Chapman P. Phase I study of PLX4032: Proof of concept for V600EBRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27:15s.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

