Comparison of darbepoetin alfa dosed weekly (QW) vs. extended dosing schedule (EDS) in the treatment of anemia in patients receiving multicycle chemotherapy in a randomized, phase 2, open-label trial
- PMID: 20973982
- PMCID: PMC2988026
- DOI: 10.1186/1471-2407-10-581
Comparison of darbepoetin alfa dosed weekly (QW) vs. extended dosing schedule (EDS) in the treatment of anemia in patients receiving multicycle chemotherapy in a randomized, phase 2, open-label trial
Abstract
Background: Chemotherapy-induced anemia (CIA) is responsive to treatment with erythropoiesis-stimulating agents (ESAs) such as darbepoetin alfa. Administration of ESAs on a synchronous schedule with chemotherapy administration could benefit patients by reducing clinic visits and potentially enhancing on-time chemotherapy delivery.
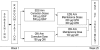
Methods: This phase 2, 25-week, open-label study evaluated the noninferiority of darbepoetin alfa administered weekly vs. as an extended dosing schedule (every 2 or 3 weeks) in patients with CIA. Patients were randomized 1:1 to an extended dosing schedule (EDS: darbepoetin alfa 300 μg Q2W if chemotherapy was QW, Q2W, or Q4W or darbepoetin alfa 500 μg Q3W if chemotherapy was Q3W) or weekly (150 μg QW regardless of chemotherapy schedule). Stratification factors included chemotherapy cycle length, screening hemoglobin (<10 g/dL vs. ≥10 g/dL), and tumor type (lung/gynecological vs. other nonmyeloid malignancies). The primary endpoint was change in hemoglobin from baseline to Week 13.
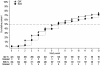
Results: Seven hundred fifty-two patients (374 QW patients; 378 EDS patients) received ≥1 dose of darbepoetin alfa and were included in the analysis. Demographics and disease state were similar between groups. Seventy-one percent of patients in the EDS group and 76% in the QW group achieved the target hemoglobin of ≥11.0 g/dL. There was a minimal difference in the primary endpoint of mean change in hemoglobin (baseline to Week 13) between the QW and the EDS groups (-0.04 g/dL; 95% confidence interval: -0.26, 0.17 g/dL). The upper limit of the 95% confidence interval was less than the prespecified limit of <0.75 g/dL, supporting noninferiority of the EDS dosing schedule. Reported adverse events were similar between groups. A slight increase in transfusions was reported in the QW group.
Conclusion: Darbepoetin alfa, when administered synchronously with chemotherapy, on an EDS appears to be similarly efficacious to darbepoetin alfa weekly dosing with no unexpected adverse events. This study provides prospective data on how multiple dosing regimens available with darbepoetin alfa can be synchronized with chemotherapy administered across a range of dosing schedules.
Trial registration: ClinicalTrials.gov Identifier NCT00144131.
Figures

Similar articles
-
Darbepoetin alfa for treating chemotherapy-induced anemia in patients with a baseline hemoglobin level < 10 g/dL versus > or = 10 g/dL: an exploratory analysis from a randomized, double-blind, active-controlled trial.BMC Cancer. 2009 Sep 3;9:311. doi: 10.1186/1471-2407-9-311. BMC Cancer. 2009. PMID: 19728887 Free PMC article. Clinical Trial.
-
Darbepoetin alfa administered every three weeks is effective for the treatment of chemotherapy-induced anemia.Oncologist. 2006 Apr;11(4):409-17. doi: 10.1634/theoncologist.11-4-409. Oncologist. 2006. PMID: 16614237
-
Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: the PROMPT study.Clin Nephrol. 2005 Aug;64(2):113-23. doi: 10.5414/cnp64113. Clin Nephrol. 2005. PMID: 16114787 Clinical Trial.
-
Extended-dosage-interval regimens of erythropoietic agents in chemotherapy-induced anemia.Am J Health Syst Pharm. 2007 Dec 15;64(24):2547-56. doi: 10.2146/ajhp070018. Am J Health Syst Pharm. 2007. PMID: 18056942 Review.
-
Optimizing the dose and schedule of darbepoetin alfa in patients with chemotherapy-induced anemia.Oncology (Williston Park). 2006 Jul;20(8 Suppl 6):29-32. Oncology (Williston Park). 2006. PMID: 16925109 Review.
Cited by
-
Erythropoietin and cancer: the unintended consequences of anemia correction.Front Immunol. 2014 Nov 11;5:563. doi: 10.3389/fimmu.2014.00563. eCollection 2014. Front Immunol. 2014. PMID: 25426117 Free PMC article. Review.
References
-
- Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19(11):2875–2882. - PubMed
-
- Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E, Taylor K, Belch A, Altes A, Martinelli G. et al.Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122(3):394–403. doi: 10.1046/j.1365-2141.2003.04448.x. - DOI - PubMed
-
- Vadhan-Raj S, Mirtsching B, Charu V, Terry D, Rossi G, Tomita D, McGuire WP. Assessment of hematologic effects and fatigue in cancer patients with chemotherapy-induced anemia given darbepoetin alfa every two weeks. J Supp Oncol. 2003;1(2):131–138. - PubMed
-
- Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, Siena S, Gateley J, Tomita D, Colowick AB. et al.Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Nat Cancer Inst. 2002;94(16):1211–1220. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

