Increased spinal prodynorphin gene expression in reinflammation-associated hyperalgesia after neonatal inflammatory insult
- PMID: 20973986
- PMCID: PMC2978219
- DOI: 10.1186/1471-2202-11-139
Increased spinal prodynorphin gene expression in reinflammation-associated hyperalgesia after neonatal inflammatory insult
Abstract
Background: Neuroplasticity induced by neonatal inflammation is the consequence of a combination of activity-dependent changes in neurons. We investigated neuronal sensitivity to a noxious stimulus in a rat model of neonatal hind-paw peripheral inflammation and assessed changes in pain behaviour at the physiological and molecular levels after peripheral reinflammation in adulthood.
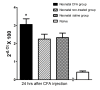
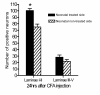
Results: A decrease in paw withdrawal latency (PWL) after a heat stimulus was documented in rats that received inflammatory injections in their left hind paws on postnatal day one (P1) and a reinflammation stimulus at postnatal 6-8 weeks of age, compared with normal rats. An increase in the expression of the prodynorphin (proDYN) gene was noted after reinflammation in the spinal cord ipsilateral to the afferents of the neonatally treated hind paw. The involvement of the activation of extracellular signal-regulated kinases (ERK) in peripheral inflammatory pain hypersensitivity was evidenced evident by the increase in phospho-ERK (pERK) activity after reinflammation.
Conclusions: Our results indicate that peripheral inflammation in neonates can permanently alter the pain processing pathway during the subsequent sensory stimulation of the region. Elucidation of the mechanism underlying the developing pain circuitry will provide new insights into the understanding of the early pain behaviours and the subsequent adaptation to pain.
Figures


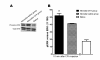
References
-
- Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989;36:795–822. - PubMed
-
- Ling QD, Ruda MA. Soc Neurosci Abstr. L. A., USA; 1998. Neonatal persistent pain alters spinal neural circuitry; p. 386.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

