Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits
- PMID: 20974008
- PMCID: PMC2976742
- DOI: 10.1186/1475-925X-9-63
Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits
Abstract
Background: Recent studies have shown the potential suitability of magnesium alloys as biodegradable implants. The aim of the present study was to compare the soft tissue biocompatibility of MgCa0.8 and commonly used surgical steel in vivo.
Methods: A biodegradable magnesium calcium alloy (MgCa0.8) and surgical steel (S316L), as a control, were investigated. Screws of identical geometrical conformation were implanted into the tibiae of 40 rabbits for a postoperative follow up of two, four, six and eight weeks. The tibialis cranialis muscle was in direct vicinity of the screw head and thus embedded in paraffin and histologically and immunohistochemically assessed. Haematoxylin and eosin staining was performed to identify macrophages, giant cells and heterophil granulocytes as well as the extent of tissue fibrosis and necrosis. Mouse anti-CD79α and rat anti-CD3 monoclonal primary antibodies were used for B- and T-lymphocyte detection. Evaluation of all sections was performed by applying a semi-quantitative score.
Results: Clinically, both implant materials were tolerated well. Histology revealed that a layer of fibrous tissue had formed between implant and overlying muscle in MgCa0.8 and S316L, which was demarcated by a layer of synoviocyte-like cells at its interface to the implant. In MgCa0.8 implants cavities were detected within the fibrous tissue, which were surrounded by the same kind of cell type. The thickness of the fibrous layer and the amount of tissue necrosis and cellular infiltrations gradually decreased in S316L. In contrast, a decrease could only be noted in the first weeks of implantation in MgCa0.8, whereas parameters were increasing again at the end of the observation period. B-lymphocytes were found more often in MgCa0.8 indicating humoral immunity and the presence of soluble antigens. Conversely, S316L displayed a higher quantity of T-lymphocytes.
Conclusions: Moderate inflammation was detected in both implant materials and resolved to a minimum during the first weeks indicating comparable biocompatibility for MgCa0.8 and S316L. Thus, the application of MgCa0.8 as biodegradable implant material seems conceivable. Since the inflammatory parameters were re-increasing at the end of the observation period in MgCa0.8 it is important to observe the development of inflammation over a longer time period in addition to the present study.
Figures
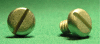








References
-
- Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

