Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart
- PMID: 20974150
- PMCID: PMC3019252
- DOI: 10.1016/j.yjmcc.2010.10.019
Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart
Abstract
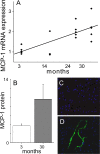
Diastolic dysfunction in the aging heart is a grave condition that challenges the life and lifestyle of a growing segment of our population. This report seeks to examine the role and interrelationship of inflammatory dysregulation in interstitial myocardial fibrosis and progressive diastolic dysfunction in aging mice. We studied a population of C57BL/6 mice that developed progressive diastolic dysfunction over 30 months of life. This progressive dysfunction was associated with increasing infiltration of CD45(+) fibroblasts of myeloid origin. In addition, increased rates of collagen expression as measured by cellular procollagen were apparent in the heart as a function of age. These cellular and functional changes were associated with progressive increases in mRNA for MCP-1 and IL-13, which correlated both temporally and quantitatively with changes in fibrosis and cellular procollagen levels. MCP-1 protein was also increased and found to be primarily in the venular endothelium. Protein assays also demonstrated elevation of IL-4 and IL-13 suggesting a shift to a Th2 phenotype in the aging heart. In vitro studies demonstrated that IL-13 markedly enhanced monocyte-fibroblast transformation. Our results indicate that immunoinflammatory dysregulation in the aging heart induces progressive MCP-1 production and an increased shift to a Th2 phenotype paralleled by an associated increase in myocardial interstitial fibrosis, cellular collagen synthesis, and increased numbers of CD45(+) myeloid-derived fibroblasts that contain procollagen. The temporal association and functional correlations suggest a causative relationship between age-dependent immunoinflammatory dysfunction, fibrosis and diastolic dysfunction.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures




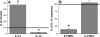
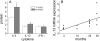
References
-
- Vanoverschelde JJ, Essamri B, Vanbutsele R, d'Hondt A, Cosyns JR, Detry JR, et al. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol. 1993;74:2225–33. - PubMed
-
- Luchi RJ, Taffet GE, Teasdale TA. Congestive heart failure in the elderly. J Am Geriatr Soc. 1991;39:810–25. - PubMed
-
- Taffet GE, Teasdale TA, Bleyer AJ, Kutka NJ, Luchi RJ. Survival of elderly men with congestive heart failure. Age Ageing. 1992;21:49–55. - PubMed
-
- Lindenfeld J, Krause-Steinrauf H, Salerno J. Where are all the women with heart failure? J Am Coll Cardiol. 1997;30:1417–9. - PubMed
-
- Ezekowitz JA, Lee DS, Tu JV, Newman AM, McAlister FA. Comparison of one-year outcome (death and rehospitalization) in hospitalized heart failure patients with left ventricular ejection fraction >50% versus those with ejection fraction <50% Am J Cardiol. 2008;102:79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

