Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction
- PMID: 20974230
- PMCID: PMC3010433
- DOI: 10.1016/j.neuroscience.2010.10.048
Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction
Abstract
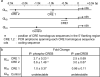
The prefrontal cortex is highly vulnerable to traumatic brain injury (TBI) and its structural and/or functional alterations as a result of TBI can give rise to persistent working memory (WM) dysfunction. Using a rodent model of TBI, we have described profound WM deficits following TBI that are associated with increases in prefrontal catecholamine (both dopamine and norepinephrine) content. In this study, we examined if enhanced norepinephrine signaling contributes to TBI-associated WM dysfunction. We demonstrate that administration of α1 adrenoceptor antagonists, but not α2A agonist, at 14 days post-injury significantly improved WM performance. mRNA analysis revealed increased levels of α1A, but not α1B or α1D, adrenoceptor in the medial prefrontal cortex (mPFC) of brain-injured rats. As α1A and 1B adrenoceptor promoters contain putative cAMP response element (CRE) sequences, we therefore examined if CRE-binding protein (CREB) actively engages these sequences in order to increase receptor gene transcription following TBI. Our results show that the phosphorylation of CREB is enhanced in the mPFC at time points during which increased α1A mRNA expression was observed. Chromatin immunoprecipitation (ChIP) assays using mPFC tissue from injured animals indicated increased phospho-CREB binding to the CRE sites of α1A, but not α1B, promoter compared to that observed in uninjured controls. To address the translatability of our findings, we tested the efficacy of the FDA-approved α1 antagonist Prazosin and observed that this drug improves WM in injured animals. Taken together, these studies suggest that enhanced CREB-mediated expression of α1 adrenoceptor contributes to TBI-associated WM dysfunction, and therapies aimed at reducing α1 signaling may be useful in the treatment of TBI-associated WM deficits in humans.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
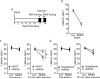
 Significant interaction between trial number and treatment. *Significant difference in latency between location and match trial.
Significant interaction between trial number and treatment. *Significant difference in latency between location and match trial.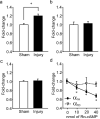



 Significant interaction between trial number and treatment. *Significant difference in latency between location and matching trial.
Significant interaction between trial number and treatment. *Significant difference in latency between location and matching trial.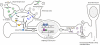
Similar articles
-
Altered regulation of protein kinase a activity in the medial prefrontal cortex of normal and brain-injured animals actively engaged in a working memory task.J Neurotrauma. 2015 Jan 15;32(2):139-48. doi: 10.1089/neu.2014.3487. Epub 2014 Nov 13. J Neurotrauma. 2015. PMID: 25027811 Free PMC article.
-
Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism.Neuroscience. 2009 Mar 17;159(2):483-91. doi: 10.1016/j.neuroscience.2008.12.050. Epub 2009 Jan 3. Neuroscience. 2009. PMID: 19167462 Free PMC article.
-
Gene expressions and mechanical functions of α1-adrenoceptor subtypes in mouse ureter.World J Urol. 2009 Dec;27(6):775-80. doi: 10.1007/s00345-009-0396-y. World J Urol. 2009. PMID: 19259685
-
The pharmacology of α1-adrenoceptor subtypes.Eur J Pharmacol. 2019 Jul 15;855:305-320. doi: 10.1016/j.ejphar.2019.04.047. Epub 2019 May 5. Eur J Pharmacol. 2019. PMID: 31067439 Review.
-
α-1 Adrenergic receptor signaling linked to memory dysfunction following brain trauma.Neurosurgery. 2011 Apr;68(4):N16-8. doi: 10.1227/01.neu.0000395791.89648.93. Neurosurgery. 2011. PMID: 21792097 Review. No abstract available.
Cited by
-
Effects of a Serotonin Receptor Peptide on Behavioral Pattern Separation in Sham- vs. Mild Traumatic Brain Injured Rats.Endocrinol Diabetes Metab J. 2024 Jun;8(2):10.31038/edmj.2024821. doi: 10.31038/edmj.2024821. Epub 2024 Jun 24. Endocrinol Diabetes Metab J. 2024. PMID: 39822258 Free PMC article.
-
Noradrenergic α1-Adrenoceptor Actions in the Primate Dorsolateral Prefrontal Cortex.J Neurosci. 2019 Apr 3;39(14):2722-2734. doi: 10.1523/JNEUROSCI.2472-18.2019. Epub 2019 Feb 12. J Neurosci. 2019. PMID: 30755491 Free PMC article.
-
Continuous Infusion of Phenelzine, Cyclosporine A, or Their Combination: Evaluation of Mitochondrial Bioenergetics, Oxidative Damage, and Cytoskeletal Degradation following Severe Controlled Cortical Impact Traumatic Brain Injury in Rats.J Neurotrauma. 2018 Jun 1;35(11):1280-1293. doi: 10.1089/neu.2017.5353. Epub 2018 Mar 27. J Neurotrauma. 2018. PMID: 29336204 Free PMC article.
-
Guanfacine's mechanism of action in treating prefrontal cortical disorders: Successful translation across species.Neurobiol Learn Mem. 2020 Dec;176:107327. doi: 10.1016/j.nlm.2020.107327. Epub 2020 Oct 17. Neurobiol Learn Mem. 2020. PMID: 33075480 Free PMC article. Review.
-
Methylphenidate Treatment of Cognitive Dysfunction in Adults After Mild to Moderate Traumatic Brain Injury: Rationale, Efficacy, and Neural Mechanisms.Front Neurol. 2019 Sep 12;10:925. doi: 10.3389/fneur.2019.00925. eCollection 2019. Front Neurol. 2019. PMID: 31572283 Free PMC article. Review.
References
-
- Arnsten AF, Goldman-Rakic PS. (Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. - PubMed
-
- Arnsten AF, Li BM. (Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. - PubMed
-
- Autelitano DJ, Woodcock EA. (Selective activation of alpha1A-adrenergic receptors in neonatal cardiac myocytes is sufficient to cause hypertrophy and differential regulation of alpha1-adrenergic receptor subtype mRNAs. J Mol Cell Cardiol. 1998;30:1515–1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

