Selective macrophage ascorbate deficiency suppresses early atherosclerosis
- PMID: 20974251
- PMCID: PMC3014415
- DOI: 10.1016/j.freeradbiomed.2010.10.702
Selective macrophage ascorbate deficiency suppresses early atherosclerosis
Abstract
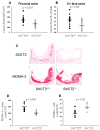
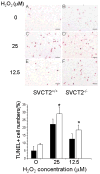
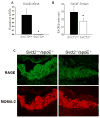
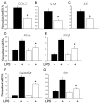
To test whether severe ascorbic acid deficiency in macrophages affects progression of early atherosclerosis, we used fetal liver cell transplantation to generate atherosclerosis-prone apolipoprotein E-deficient (apoE(-/-)) mice that selectively lacked the ascorbate transporter (SVCT2) in hematopoietic cells, including macrophages. After 13 weeks of chow diet, apoE(-/-) mice lacking the SVCT2 in macrophages had surprisingly less aortic atherosclerosis, decreased lesion macrophage numbers, and increased macrophage apoptosis compared to control-transplanted mice. Serum lipid levels were similar in both groups. Peritoneal macrophages lacking the SVCT2 had undetectable ascorbate; increased susceptibility to H(2)O(2)-induced mitochondrial dysfunction and apoptosis; decreased expression of genes for COX-2, IL1β, and IL6; and decreased lipopolysaccharide-stimulated NF-κB and antiapoptotic gene expression. These changes were associated with decreased expression of both the receptor for advanced glycation end products and HIF-1α, either or both of which could have been the proximal cause of decreased macrophage activation and apoptosis in ascorbate-deficient macrophages.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. - PubMed
-
- Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S35–S40. - PubMed
-
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. - PMC - PubMed
-
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL065709/HL/NHLBI NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- HL065405/HL/NHLBI NIH HHS/United States
- P50 GM015431/GM/NIGMS NIH HHS/United States
- R01 HL057986/HL/NHLBI NIH HHS/United States
- R01 HL065709/HL/NHLBI NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- GM15431/GM/NIGMS NIH HHS/United States
- R01 HL086988/HL/NHLBI NIH HHS/United States
- R01 DK050435/DK/NIDDK NIH HHS/United States
- HL086988/HL/NHLBI NIH HHS/United States
- DK020593/DK/NIDDK NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- R01 HL065405/HL/NHLBI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- DK050435/DK/NIDDK NIH HHS/United States
- HL057986/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

