Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans
- PMID: 20974306
- PMCID: PMC3223387
- DOI: 10.1016/j.vaccine.2010.10.023
Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans
Abstract
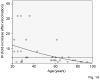
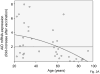
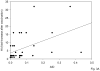
We have evaluated the serum response to seasonal influenza vaccination in subjects of different ages and associated this with the specific B cell response to the vaccine in vitro. Although the serum response has previously been shown to decrease with age, this has largely been associated to decreased T cell functions. Our results show that in response to the vaccine, the specific response of B cells in vitro, as measured by AID (activation-induced cytidine deaminase), the in vivo serum HI (hemagglutination inhibition) response, and the in vivo generation of switch memory B cells are decreased with age, as evaluated in the same subjects. This is the first report to demonstrate that intrinsic B cell defects with age contribute to reduced antibody responses to the influenza vaccine. The level of AID in response to CpG before vaccination can also predict the robustness of the vaccine response. These results could contribute to developing more effective vaccines to protect the elderly as well as identifying those most at risk.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures






References
-
- Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998 Jul;178(1):53–60. - PubMed
-
- Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity Addressing immune senescence to ensure healthy ageing. Vaccine. Apr 1; - PubMed
-
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007 Oct;7(10):658–66. - PubMed
-
- Centers for Disease Control and Prevention. Racial/ethnic disparities in influenza and pneumococcal vaccination levels among persons aged > or = 65 years in the United States, 1989–2001. MMWR Morb Mortal Wkly Rep. 2003;52:958–62. - PubMed
-
- Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995 Oct 1;123(7):518–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

