Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity
- PMID: 20974672
- PMCID: PMC3035083
- DOI: 10.1182/blood-2010-06-290171
Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity
Abstract
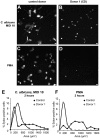
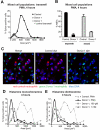
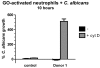
The granule enzyme myeloperoxidase (MPO) plays an important role in neutrophil antimicrobial responses. However, the severity of immunodeficiency in patients carrying mutations in MPO is variable. Serious microbial infections, especially with Candida species, have been observed in a subset of completely MPO-deficient patients. Here we show that neutrophils from donors who are completely deficient in MPO fail to form neutrophil extracellular traps (NETs), indicating that MPO is required for NET formation. In contrast, neutrophils from partially MPO-deficient donors make NETs, and pharmacological inhibition of MPO only delays and reduces NET formation. Extracellular products of MPO do not rescue NET formation, suggesting that MPO acts cell-autonomously. Finally, NET-dependent inhibition of Candida albicans growth is compromised in MPO-deficient neutrophils. The inability to form NETs may contribute in part to the host defense defects observed in completely MPO-deficient individuals.
Figures


References
-
- Schultz J, Kaminker K. Myeloperoxidase of leucocyte of normal human blood. 1. Content and localization. Arch Biochem Biophys. 1962;96(3):465–467. - PubMed
-
- Harrison JE, Schultz J. Studies on chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251(5):1371–1374. - PubMed
-
- Klebanoff SJ. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972;12(2):170–196. - PubMed
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77(5):598–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

