Airway peroxidases catalyze nitration of the {beta}2-agonist salbutamol and decrease its pharmacological activity
- PMID: 20974700
- PMCID: PMC3033722
- DOI: 10.1124/jpet.110.170027
Airway peroxidases catalyze nitration of the {beta}2-agonist salbutamol and decrease its pharmacological activity
Abstract
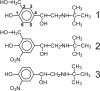
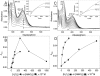
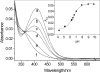
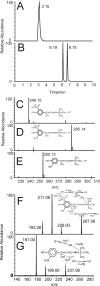
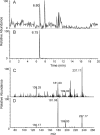
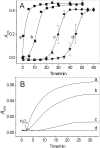
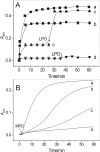
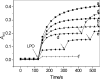
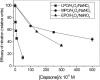
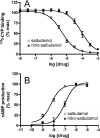
β(2)-agonists are the most effective bronchodilators for the rapid relief of asthma symptoms, but for unclear reasons, their effectiveness may be decreased during severe exacerbations. Because peroxidase activity and nitrogen oxides are increased in the asthmatic airway, we examined whether salbutamol, a clinically important β(2)-agonist, is subject to potentially inactivating nitration. When salbutamol was exposed to myeloperoxidase, eosinophil peroxidase or lactoperoxidase in the presence of hydrogen peroxide (H(2)O(2)) and nitrite (NO(2)(-)), both absorption spectroscopy and mass spectrometry indicated formation of a new metabolite with features expected for the nitrated drug. The new metabolites showed an absorption maximum at 410 nm and pK(a) of 6.6 of the phenolic hydroxyl group. In addition to nitrosalbutamol (m/z 285.14), a salbutamol-derived nitrophenol, formed by elimination of the formaldehyde group, was detected (m/z 255.13) by mass spectrometry. It is noteworthy that the latter metabolite was detected in exhaled breath condensates of asthma patients receiving salbutamol but not in unexposed control subjects, indicating the potential for β(2)-agonist nitration to occur in the inflamed airway in vivo. Salbutamol nitration was inhibited in vitro by ascorbate, thiocyanate, and the pharmacological agents methimazole and dapsone. The efficacy of inhibition depended on the nitrating system, with the lactoperoxidase/H(2)O(2)/NO(2)(-) being the most affected. Functionally, nitrated salbutamol showed decreased affinity for β(2)-adrenergic receptors and impaired cAMP synthesis in airway smooth muscle cells compared with the native drug. These results suggest that under inflammatory conditions associated with asthma, phenolic β(2)-agonists may be subject to peroxidase-catalyzed nitration that could potentially diminish their therapeutic efficacy.
Figures

References
-
- Abramson MJ, Walters J, Walters EH. (2003) Adverse effects of β-agonists: are they clinically relevant? Am J Respir Med 2:287–297 - PubMed
-
- Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, Town GI, Kettle AJ. (2002) Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic Biol Med 33:847–856 - PubMed
-
- Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. (2003) Oxidative and nitrosative events in asthma. Free Radic Biol Med 35:213–225 - PubMed
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745 - PubMed
-
- Bozeman PM, Learn DB, Thomas EL. (1992) Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem Pharmacol 44:553–563 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

