Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.)
- PMID: 20974734
- PMCID: PMC3003818
- DOI: 10.1093/jxb/erq312
Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.)
Abstract
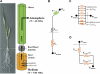
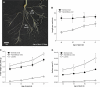
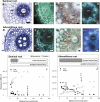
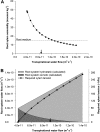
Prior to an assessment of the role of aquaporins in root water uptake, the main path of water movement in different types of root and driving forces during day and night need to be known. In the present study on hydroponically grown barley (Hordeum vulgare L.) the two main root types of 14- to 17-d-old plants were analysed for hydraulic conductivity in dependence of the main driving force (hydrostatic, osmotic). Seminal roots contributed 92% and adventitious roots 8% to plant water uptake. The lower contribution of adventitious compared with seminal roots was associated with a smaller surface area and number of roots per plant and a lower axial hydraulic conductance, and occurred despite a less-developed endodermis. The radial hydraulic conductivity of the two types of root was similar and depended little on the prevailing driving force, suggesting that water uptake occurred along a pathway that involved crossing of membrane(s). Exudation experiments showed that osmotic forces were sufficient to support night-time transpiration, yet transpiration experiments and cuticle permeance data questioned the significance of osmotic forces. During the day, 90% of water uptake was driven by a tension of about -0.15 MPa.
Figures


References
-
- Brundrett MC, Enstone DE, Peterson CA. A berberine-aniline blue staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma. 1988;146:133–142.
-
- Brundrett MC, Kendrick B, Peterson CA. Efficient lipid staining in plant material with Sudan red 7B or fluorol yellow 088 in polyethylene glycol-glycerol. Biotechnology and Histochemistry. 1991;66:111–116. - PubMed

