Rapid topology probing using fluorescence spectroscopy in planar lipid bilayer: the pore-forming mechanism of the toxin Cry1Aa of Bacillus thuringiensis
- PMID: 20974771
- PMCID: PMC2964520
- DOI: 10.1085/jgp.200910347
Rapid topology probing using fluorescence spectroscopy in planar lipid bilayer: the pore-forming mechanism of the toxin Cry1Aa of Bacillus thuringiensis
Abstract
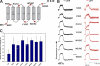
Pore-forming toxins, many of which are pathogenic to humans, are highly dynamic proteins that adopt a different conformation in aqueous solution than in the lipid environment of the host membrane. Consequently, their crystal structures obtained in aqueous environment do not reflect the active conformation in the membrane, making it difficult to deduce the molecular determinants responsible for pore formation. To obtain structural information directly in the membrane, we introduce a fluorescence technique to probe the native topology of pore-forming toxins in planar lipid bilayers and follow their movement during pore formation. Using a Förster resonance energy transfer (FRET) approach between site-directedly labeled proteins and an absorbing compound (dipicrylamine) in the membrane, we simultaneously recorded the electrical current and fluorescence emission in horizontal planar lipid bilayers formed in plastic chips. With this system, we mapped the topology of the pore-forming domain of Cry1Aa, a biological pesticide from Bacillus thuringiensis, by determining the location of the loops between its seven α helices. We found that the majority of the toxins initially traverse from the cis to the trans leaflet of the membrane. Comparing the topologies of Cry1Aa in the active and inactive state in order to identify the pore-forming mechanism, we established that only the α3-α4 hairpin translocates through the membrane from the trans to the cis leaflet, whereas all other positions remained constant. As toxins are highly dynamic proteins, populations that differ in conformation might be present simultaneously. To test the presence of different populations, we designed double-FRET experiments, where a single donor interacts with two acceptors with very different kinetics (dipicrylamine and oxonol). Due to the nonlinear response of FRET and the dynamic change of the acceptor distribution, we can deduce the distribution of the acceptors in the membrane from the time course of the donor fluorescence. We found that Cry1Aa is present on both membrane leaflets.
Figures



Similar articles
-
Mutations in domain I interhelical loops affect the rate of pore formation by the Bacillus thuringiensis Cry1Aa toxin in insect midgut brush border membrane vesicles.Appl Environ Microbiol. 2009 Jun;75(12):3842-50. doi: 10.1128/AEM.02924-08. Epub 2009 Apr 17. Appl Environ Microbiol. 2009. PMID: 19376918 Free PMC article.
-
The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis delta-endotoxin are consistent with an "umbrella-like" structure of the pore.Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12289-94. doi: 10.1073/pnas.95.21.12289. Proc Natl Acad Sci U S A. 1998. PMID: 9770479 Free PMC article.
-
Importance of polarity of the α4-α5 loop residue-Asn(166) in the pore-forming domain of the Bacillus thuringiensis Cry4Ba toxin: implications for ion permeation and pore opening.Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):319-27. doi: 10.1016/j.bbamem.2013.10.002. Epub 2013 Oct 10. Biochim Biophys Acta. 2014. PMID: 24120447
-
Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins.Toxins (Basel). 2014 Sep 16;6(9):2732-70. doi: 10.3390/toxins6092732. Toxins (Basel). 2014. PMID: 25229189 Free PMC article. Review.
-
Structural and functional analysis of the pre-pore and membrane-inserted pore of Cry1Ab toxin.J Invertebr Pathol. 2006 Jul;92(3):172-7. doi: 10.1016/j.jip.2006.02.008. Epub 2006 Jun 14. J Invertebr Pathol. 2006. PMID: 16777138 Review.
Cited by
-
Acquiring snapshots of the orientation of trans-membrane protein domains using a hybrid FRET pair.FEBS Lett. 2015 Apr 2;589(8):885-9. doi: 10.1016/j.febslet.2015.02.030. Epub 2015 Mar 3. FEBS Lett. 2015. PMID: 25747388 Free PMC article.
-
Channel Formation in Cry Toxins: An Alphafold-2 Perspective.Int J Mol Sci. 2023 Nov 27;24(23):16809. doi: 10.3390/ijms242316809. Int J Mol Sci. 2023. PMID: 38069132 Free PMC article.
-
Structure of anthrax lethal toxin prepore complex suggests a pathway for efficient cell entry.J Gen Physiol. 2016 Oct;148(4):313-24. doi: 10.1085/jgp.201611617. J Gen Physiol. 2016. PMID: 27670897 Free PMC article.
-
Do lipids show state-dependent affinity to the voltage-gated potassium channel KvAP?J Biol Chem. 2014 Jun 6;289(23):16452-61. doi: 10.1074/jbc.M113.537134. Epub 2014 Apr 17. J Biol Chem. 2014. PMID: 24742679 Free PMC article.
-
The 2018 correlative microscopy techniques roadmap.J Phys D Appl Phys. 2018 Nov 7;51(44):443001. doi: 10.1088/1361-6463/aad055. Epub 2018 Aug 31. J Phys D Appl Phys. 2018. PMID: 30799880 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

