Variation in methods of predicting adult height for children with idiopathic short stature
- PMID: 20974789
- PMCID: PMC3793344
- DOI: 10.1542/peds.2009-3649
Variation in methods of predicting adult height for children with idiopathic short stature
Abstract
Objective: Recombinant human growth hormone (GH) is approved for treatment of children with idiopathic short stature, and endocrinologists often depend on algorithms to predict adult height. Because algorithm performance often is included in treatment decisions, we sought to evaluate agreement among height prediction formulas.
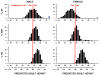
Methods: We identified 3 commonly used algorithms for height prediction, the Bayley-Pinneau, Roche-Wainer-Thissen, and Khamis-Roche methods. We constructed simulated samples of children with typical distributions of ages, heights, weights, bone ages, and parental heights seen in patients with idiopathic short stature, and we applied the algorithms to the simulated sample to determine whether predicted adult height was <160 cm for boys or <150 cm for girls (<1.2nd height percentiles for adults).
Results: We found substantial disagreement among algorithms in the proportions of simulated cases with predicted adult heights of <1.2nd percentile, a cutoff value that may influence GH treatment decisions. With the Bayley-Pinneau formula, 43% of boys and 81% of girls had predicted adult heights below this threshold; with the Khamis-Roche method, only 3% of boys and 0.2% of girls had predicted heights of <1.2nd percentile. Roche-Wainer-Thissen predictions were between those values. Overall agreement of the methods was poor (κ = 0.21) for boys and negative for girls.
Conclusions: Wide variation exists among formulas used to predict adult heights. Because these algorithms may be used in decisions regarding whether to initiate GH treatment and assessment of the efficacy of GH in research trials, it is important for parents, pediatricians, and investigators to recognize the considerable variation involved in height predictions.
Figures
References
-
- (FDA) FaDA. [Accessed 10/31/2005];FDA Talk Paper: FDA Approves Humatrope for Short Stature. http://www.fda.gov/bbs/topics/ANSWERS/2003/ANS01242.html. Published 2003.
-
- Cuttler L, Silvers JB. Growth hormone treatment for idiopathic short stature: implications for practice and policy. Arch Pediatr Adolesc Med. 2004;158(2):108–110. - PubMed
-
- Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(7):3140–3148. - PubMed
-
- Wit JM, Rekers-Mombarg LT, Cutler GB, Crowe B, Beck TJ, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect. J Pediatr. 2005;146(1):45–53. - PubMed
-
- Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007;(3):CD004440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

