Quality control for unfolded proteins at the plasma membrane
- PMID: 20974815
- PMCID: PMC3003321
- DOI: 10.1083/jcb.201006012
Quality control for unfolded proteins at the plasma membrane
Abstract
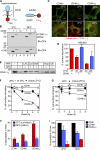
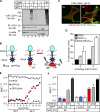
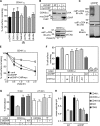
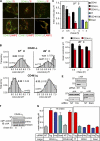
Cellular protein homeostasis profoundly depends on the disposal of terminally damaged polypeptides. To demonstrate the operation and elucidate the molecular basis of quality control of conformationally impaired plasma membrane (PM) proteins, we constructed CD4 chimeras containing the wild type or a temperature-sensitive bacteriophage λ domain in their cytoplasmic region. Using proteomic, biochemical, and genetic approaches, we showed that thermal unfolding of the λ domain at the PM provoked the recruitment of Hsp40/Hsc70/Hsp90 chaperones and the E2-E3 complex. Mixed-chain polyubiquitination, monitored by bioluminescence resonance energy transfer and immunoblotting, is responsible for the nonnative chimera-accelerated internalization, impaired recycling, and endosomal sorting complex required for transport-dependent lysosomal degradation. A similar paradigm prevails for mutant dopamine D4.4 and vasopressin V2 receptor removal from the PM. These results outline a peripheral proteostatic mechanism in higher eukaryotes and its potential contribution to the pathogenesis of a subset of conformational diseases.
Figures



References
-
- Barriere H., Lukacs G.L. 2008. Analysis of endocytic trafficking by single-cell fluorescence ratio imaging. Curr. Protoc. Cell Biol. Chapter 15:Unit 15.13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

