Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps
- PMID: 20974816
- PMCID: PMC3003309
- DOI: 10.1083/jcb.201006052
Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps
Abstract
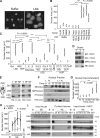
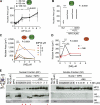
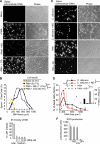
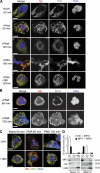
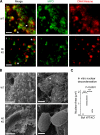
Neutrophils release decondensed chromatin termed neutrophil extracellular traps (NETs) to trap and kill pathogens extracellularly. Reactive oxygen species are required to initiate NET formation but the downstream molecular mechanism is unknown. We show that upon activation, neutrophil elastase (NE) escapes from azurophilic granules and translocates to the nucleus, where it partially degrades specific histones, promoting chromatin decondensation. Subsequently, myeloperoxidase synergizes with NE in driving chromatin decondensation independent of its enzymatic activity. Accordingly, NE knockout mice do not form NETs in a pulmonary model of Klebsiella pneumoniae infection, which suggests that this defect may contribute to the immune deficiency of these mice. This mechanism provides for a novel function for serine proteases and highly charged granular proteins in the regulation of chromatin density, and reveals that the oxidative burst induces a selective release of granular proteins into the cytoplasm through an unknown mechanism.
Figures

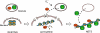
References
-
- Ancliff P.J., Gale R.E., Liesner R., Hann I.M., Linch D.C. 2001. Mutations in the ELA2 gene encoding neutrophil elastase are present in most patients with sporadic severe congenital neutropenia but only in some patients with the familial form of the disease. Blood. 98:2645–2650 10.1182/blood.V98.9.2645 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

