Lipoproteins of bacterial pathogens
- PMID: 20974828
- PMCID: PMC3028857
- DOI: 10.1128/IAI.00682-10
Lipoproteins of bacterial pathogens
Abstract
Bacterial lipoproteins are a set of membrane proteins with many different functions. Due to this broad-ranging functionality, these proteins have a considerable significance in many phenomena, from cellular physiology through cell division and virulence. Here we give a general overview of lipoprotein biogenesis and highlight examples of the roles of lipoproteins in bacterial disease caused by a selection of medically relevant Gram-negative and Gram-positive pathogens: Mycobacterium tuberculosis, Streptococcus pneumoniae, Borrelia burgdorferi, and Neisseria meningitidis. Lipoproteins have been shown to play key roles in adhesion to host cells, modulation of inflammatory processes, and translocation of virulence factors into host cells. As such, a number of lipoproteins have been shown to be potential vaccines. This review provides a summary of some of the reported roles of lipoproteins and of how this knowledge has been exploited in some cases for the generation of novel countermeasures to bacterial diseases.
Figures
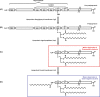
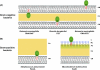
References
-
- Alloing, G., P. de Philip, and J. P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. - PubMed
-
- Arenas, J., A. Abel, S. Sanchez, B. Alcala, M. T. Criado, and C. M. Ferreiros. 2006. Locus NMB0035 codes for a 47-kDa surface-accessible conserved antigen in Neisseria. Int. Microbiol. 9:273-280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

