The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria
- PMID: 20974832
- PMCID: PMC3019871
- DOI: 10.1128/IAI.00937-10
The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria
Abstract
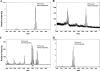
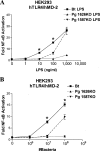
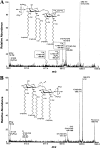
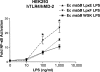
The human symbiont Bacteroides thetaiotaomicron promotes intestinal function and health, whereas the phylogenetically related pathogen Porphyromonas gingivalis is associated with the chronic oral inflammatory disease periodontitis. Although both B. thetaiotaomicron and P. gingivalis synthesize lipopolysaccharides (LPS) consisting of penta-acylated, monophosphorylated lipid A in addition to immunologically silent, nonphosphorylated lipid A, they elicit strikingly distinct Toll-like receptor 4 (TLR4) responses. We show that the phosphate position of penta-acylated, monophosphorylated lipid A is a key feature for determining the differential TLR4 responses elicited by these evolutionarily related bacteria. B. thetaiotaomicron produces TLR4-stimulatory lipid A bearing a 1-phosphate, in contrast to P. gingivalis, which produces TLR4-evasive lipid A bearing a 4'-phosphate. Confirming these observations, recombinant Escherichia coli LPS containing penta-acylated, 1-phosphorylated lipid A is more TLR4 stimulatory than LPS containing 4'-phosphorylated lipid A. The specific capacity of a Gram-negative bacterium to alert or evade the host innate immune defense system through TLR4-dependent signaling is currently recognized as a critical aspect defining the relationship between the host and the bacterium. We propose that the distinct lipid A phosphate positions observed for the B. thetaiotaomicron and P. gingivalis LPS contributes to the manifestation of these bacteria as commensal or pathogen within the human host.
Figures


Similar articles
-
Identification of PGN_1123 as the Gene Encoding Lipid A Deacylase, an Enzyme Required for Toll-Like Receptor 4 Evasion, in Porphyromonas gingivalis.J Bacteriol. 2019 May 8;201(11):e00683-18. doi: 10.1128/JB.00683-18. Print 2019 Jun 1. J Bacteriol. 2019. PMID: 30782639 Free PMC article.
-
The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses.Microb Pathog. 2009 Aug;47(2):68-77. doi: 10.1016/j.micpath.2009.04.015. Epub 2009 May 19. Microb Pathog. 2009. PMID: 19460428 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition.Cell Microbiol. 2006 May;8(5):857-68. doi: 10.1111/j.1462-5822.2005.00672.x. Cell Microbiol. 2006. PMID: 16611234
-
Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A.Front Biosci. 2007 May 1;12:3795-812. doi: 10.2741/2353. Front Biosci. 2007. PMID: 17485340 Review.
-
Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system.Acta Odontol Scand. 2001 Jun;59(3):131-8. doi: 10.1080/000163501750266710. Acta Odontol Scand. 2001. PMID: 11501881 Review.
Cited by
-
Gut microbiota-based translational biomarkers to prevent metabolic syndrome via nutritional modulation.FEMS Microbiol Ecol. 2014 Feb;87(2):303-14. doi: 10.1111/1574-6941.12250. Epub 2013 Dec 12. FEMS Microbiol Ecol. 2014. PMID: 24219358 Free PMC article. Review.
-
The Xanthomonas RaxH-RaxR Two-Component Regulatory System Is Orthologous to the Zinc-Responsive Pseudomonas ColS-ColR System.Microorganisms. 2021 Jul 7;9(7):1458. doi: 10.3390/microorganisms9071458. Microorganisms. 2021. PMID: 34361895 Free PMC article.
-
CRISPR-based functional genomics in human dendritic cells.Elife. 2021 Apr 27;10:e65856. doi: 10.7554/eLife.65856. Elife. 2021. PMID: 33904395 Free PMC article.
-
Subgingival Plaque in Periodontal Health Antagonizes at Toll-Like Receptor 4 and Inhibits E-Selectin Expression on Endothelial Cells.Infect Immun. 2015 Oct 19;84(1):120-6. doi: 10.1128/IAI.00693-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483407 Free PMC article.
-
Lipid A structural modifications in extreme conditions and identification of unique modifying enzymes to define the Toll-like receptor 4 structure-activity relationship.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Nov;1862(11):1439-1450. doi: 10.1016/j.bbalip.2017.01.004. Epub 2017 Jan 17. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28108356 Free PMC article. Review.
References
-
- Bainbridge, B. W., S. R. Coats, T. T. Pham, R. A. Reife, and R. P. Darveau. 2006. Expression of a Porphyromonas gingivalis lipid A palmitylacyltransferase in Escherichia coli yields a chimeric lipid A with altered ability to stimulate interleukin-8 secretion. Cell Microbiol. 8:120-129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

