Structural modeling and electron paramagnetic resonance spectroscopy of the human Na+/H+ exchanger isoform 1, NHE1
- PMID: 20974853
- PMCID: PMC3013023
- DOI: 10.1074/jbc.M110.159202
Structural modeling and electron paramagnetic resonance spectroscopy of the human Na+/H+ exchanger isoform 1, NHE1
Abstract
We previously presented evidence that transmembrane domain (TM) IV and TM X-XI are important for inhibitor binding and ion transport by the human Na(+)/H(+) exchanger, hNHE1 (Pedersen, S. F., King, S. A., Nygaard, E. B., Rigor, R. R., and Cala, P. M. (2007) J. Biol. Chem. 282, 19716-19727). Here, we present a structural model of the transmembrane part of hNHE1 that further supports this conclusion. The hNHE1 model was based on the crystal structure of the Escherichia coli Na(+)/H(+) antiporter, NhaA, and previous cysteine scanning accessibility studies of hNHE1 and was validated by EPR spectroscopy of spin labels in TM IV and TM XI, as well as by functional analysis of hNHE1 mutants. Removal of all endogenous cysteines in hNHE1, introduction of the mutations A173C (TM IV) and/or I461C (TM XI), and expression of the constructs in mammalian cells resulted in functional hNHE1 proteins. The distance between these spin labels was ∼15 A, confirming that TM IV and TM XI are in close proximity. This distance was decreased both at pH 5.1 and in the presence of the NHE1 inhibitor cariporide. A similar TM IV·TM XI distance and a similar change upon a pH shift were found for the cariporide-insensitive Pleuronectes americanus (pa) NHE1; however, in paNHE1, cariporide had no effect on TM IV·TM XI distance. The central role of the TM IV·TM XI arrangement was confirmed by the partial loss of function upon mutation of Arg(425), which the model predicts stabilizes this arrangement. The data are consistent with a role for TM IV and TM XI rearrangements coincident with ion translocation and inhibitor binding by hNHE1.
Figures


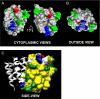

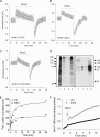




Comment in
-
Two conflicting NHE1 model structures: compatibility with experimental data and implications for the transport mechanism.J Biol Chem. 2011 May 27;286(21):le9; author reply Ie10. doi: 10.1074/jbc.L110.159202. J Biol Chem. 2011. PMID: 21602285 Free PMC article. No abstract available.
References
-
- Orlowski J., Grinstein S. (2004) Pflugers Arch. 447, 549–565 - PubMed
-
- Pedersen S. F., O'Donnell M. E., Anderson S. E., Cala P. M. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1–R25 - PubMed
-
- Wakabayashi S., Pang T., Su X., Shigekawa M. (2000) J. Biol. Chem. 275, 7942–7949 - PubMed
-
- McLean L. A., Zia S., Gorin F. A., Cala P. M. (1999) Am. J. Physiol. 276, C1025–C1037 - PubMed
-
- Pedersen S. F., King S. A., Rigor R. R., Zhuang Z., Cala P. M. (2003) Bull. Mt. Desert Island 42, 38–39
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

