Assessment of the validity of the double superhelix model for reconstituted high density lipoproteins: a combined computational-experimental approach
- PMID: 20974855
- PMCID: PMC3003414
- DOI: 10.1074/jbc.M110.187799
Assessment of the validity of the double superhelix model for reconstituted high density lipoproteins: a combined computational-experimental approach
Abstract
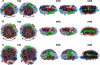
For several decades, the standard model for high density lipoprotein (HDL) particles reconstituted from apolipoprotein A-I (apoA-I) and phospholipid (apoA-I/HDL) has been a discoidal particle ∼100 Å in diameter and the thickness of a phospholipid bilayer. Recently, Wu et al. (Wu, Z., Gogonea, V., Lee, X., Wagner, M. A., Li, X. M., Huang, Y., Undurti, A., May, R. P., Haertlein, M., Moulin, M., Gutsche, I., Zaccai, G., Didonato, J. A., and Hazen, S. L. (2009) J. Biol. Chem. 284, 36605-36619) used small angle neutron scattering to develop a new model they termed double superhelix (DSH) apoA-I that is dramatically different from the standard model. Their model possesses an open helical shape that wraps around a prolate ellipsoidal type I hexagonal lyotropic liquid crystalline phase. Here, we used three independent approaches, molecular dynamics, EM tomography, and fluorescence resonance energy transfer spectroscopy (FRET) to assess the validity of the DSH model. (i) By using molecular dynamics, two different approaches, all-atom simulated annealing and coarse-grained simulation, show that initial ellipsoidal DSH particles rapidly collapse to discoidal bilayer structures. These results suggest that, compatible with current knowledge of lipid phase diagrams, apoA-I cannot stabilize hexagonal I phase particles of phospholipid. (ii) By using EM, two different approaches, negative stain and cryo-EM tomography, show that reconstituted apoA-I/HDL particles are discoidal in shape. (iii) By using FRET, reconstituted apoA-I/HDL particles show a 28-34-Å intermolecular separation between terminal domain residues 40 and 240, a distance that is incompatible with the dimensions of the DSH model. Therefore, we suggest that, although novel, the DSH model is energetically unfavorable and not likely to be correct. Rather, we conclude that all evidence supports the likelihood that reconstituted apoA-I/HDL particles, in general, are discoidal in shape.
Figures




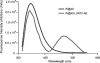
 , respectively. The W@40 and W@40:L240C-AE rHDL spectra were normalized, correcting for their proportion (1:5 with W@
, respectively. The W@40 and W@40:L240C-AE rHDL spectra were normalized, correcting for their proportion (1:5 with W@ ) in rHDL. Background fluorescence was eliminated by subtracting the emission spectrum of rHDL consisting of W@
) in rHDL. Background fluorescence was eliminated by subtracting the emission spectrum of rHDL consisting of W@ from the normalized emission spectrum of W@40 and W@40:L240C-AE rHDL. Energy transfer efficiency (E) was calculated from background corrected spectra by comparing Trp fluorescence intensities (integrated over 310–425 nm) of W@40 (donor only) and W@40:L240C-AE (donor-acceptor).
from the normalized emission spectrum of W@40 and W@40:L240C-AE rHDL. Energy transfer efficiency (E) was calculated from background corrected spectra by comparing Trp fluorescence intensities (integrated over 310–425 nm) of W@40 (donor only) and W@40:L240C-AE (donor-acceptor).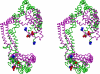

Similar articles
-
Structures of discoidal high density lipoproteins: a combined computational-experimental approach.J Biol Chem. 2010 Feb 12;285(7):4652-65. doi: 10.1074/jbc.M109.069914. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948731 Free PMC article.
-
Arrangement of apolipoprotein A-I in reconstituted high-density lipoprotein disks: an alternative model based on fluorescence resonance energy transfer experiments.Biochemistry. 2001 Apr 24;40(16):5065-74. doi: 10.1021/bi002815q. Biochemistry. 2001. PMID: 11305923
-
Double superhelix model of high density lipoprotein.J Biol Chem. 2009 Dec 25;284(52):36605-36619. doi: 10.1074/jbc.M109.039537. Epub 2009 Oct 7. J Biol Chem. 2009. PMID: 19812036 Free PMC article.
-
Comparative models for human apolipoprotein A-I bound to lipid in discoidal high-density lipoprotein particles.Biochemistry. 2002 Sep 10;41(36):10895-905. doi: 10.1021/bi020315m. Biochemistry. 2002. PMID: 12206659 Review.
-
Structural studies of discoidal lipoprotein A-I.Cell Mol Life Sci. 2001 Jun;58(7):885-93. doi: 10.1007/PL00000908. Cell Mol Life Sci. 2001. PMID: 11497237 Free PMC article. Review.
Cited by
-
A model of lipid-free apolipoprotein A-I revealed by iterative molecular dynamics simulation.PLoS One. 2015 Mar 20;10(3):e0120233. doi: 10.1371/journal.pone.0120233. eCollection 2015. PLoS One. 2015. PMID: 25793886 Free PMC article.
-
Tertiary structure of apolipoprotein A-I in nascent high-density lipoproteins.Proc Natl Acad Sci U S A. 2018 May 15;115(20):5163-5168. doi: 10.1073/pnas.1721181115. Epub 2018 Apr 30. Proc Natl Acad Sci U S A. 2018. PMID: 29712830 Free PMC article.
-
New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism.J Lipid Res. 2013 Aug;54(8):2034-2048. doi: 10.1194/jlr.R034025. Epub 2012 Dec 10. J Lipid Res. 2013. PMID: 23230082 Free PMC article. Review.
-
Structural Insights into High Density Lipoprotein: Old Models and New Facts.Front Pharmacol. 2016 Jan 12;6:318. doi: 10.3389/fphar.2015.00318. eCollection 2015. Front Pharmacol. 2016. PMID: 26793109 Free PMC article. Review.
-
The low-resolution structure of nHDL reconstituted with DMPC with and without cholesterol reveals a mechanism for particle expansion.J Lipid Res. 2013 Apr;54(4):966-83. doi: 10.1194/jlr.M032763. Epub 2013 Jan 23. J Lipid Res. 2013. PMID: 23349207 Free PMC article.
References
-
- Linsel-Nitschke P., Tall A. R. (2005) Nat. Rev. Drug Discov. 4, 193–205 - PubMed
-
- Forte T. M., Nichols A. V., Gong E. L., Levy R. I., Lux S. (1971) Biochim. Biophys. Acta 248, 381–386 - PubMed
-
- Sloop C. H., Dory L., Hamilton R., Krause B. R., Roheim P. S. (1983) J. Lipid Res. 24, 1429–1440 - PubMed
-
- Wlodawer A., Segrest J. P., Chung B. H., Chiovetti R., Jr., Weinstein J. N. (1979) FEBS Lett. 104, 231–235 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

