Decorin antagonizes Met receptor activity and down-regulates {beta}-catenin and Myc levels
- PMID: 20974860
- PMCID: PMC3009933
- DOI: 10.1074/jbc.M110.172841
Decorin antagonizes Met receptor activity and down-regulates {beta}-catenin and Myc levels
Abstract
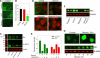
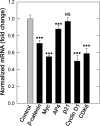
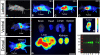
A theme emerging during the past few years is that members of the small leucine-rich proteoglycan gene family affect cell growth by interacting with multiple receptor tyrosine kinases (RTKs), mostly by a physical down-regulation of the receptors, thereby depriving tumor cells of pro-survival signals. Decorin binds and down-regulates several RTKs, including Met, the receptor for hepatocyte growth factor. Here we demonstrate that decorin blocks several biological activities mediated by the Met signaling axis, including cell scatter, evasion, and migration. These effects were mediated by a profound down-regulation of noncanonical β-catenin levels. In addition, Myc, a downstream target of β-catenin, was markedly down-regulated by decorin, whereas phosphorylation of Myc at threonine 58 was markedly induced. The latter is known to destabilize Myc and target it for proteasomal degradation. We also discovered that systemic delivery of decorin using three distinct tumor xenograft models caused down-regulation of Met and a concurrent suppression of β-catenin and Myc levels. We found that decorin protein core labeled with the near infrared dye IR800 specifically targeted the tumor cells expressing Met. Even 68-h post-injection, decorin was found to reside within the tumor xenografts with little or no binding to other tissues. Collectively, our results indicate a role for a secreted proteoglycan in suppressing the expression of key oncogenic factors required for tumor progression.
Figures




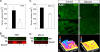

Similar articles
-
Decorin is a novel antagonistic ligand of the Met receptor.J Cell Biol. 2009 May 18;185(4):743-54. doi: 10.1083/jcb.200901129. Epub 2009 May 11. J Cell Biol. 2009. PMID: 19433454 Free PMC article.
-
Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3.J Biol Chem. 2012 Feb 17;287(8):5492-506. doi: 10.1074/jbc.M111.283499. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194599 Free PMC article.
-
Decorin as a multivalent therapeutic agent against cancer.Adv Drug Deliv Rev. 2016 Feb 1;97:174-85. doi: 10.1016/j.addr.2015.10.016. Epub 2015 Oct 30. Adv Drug Deliv Rev. 2016. PMID: 26522384 Free PMC article. Review.
-
Tumor-specific inhibitory action of decorin on different hepatoma cell lines.Cell Signal. 2019 Oct;62:109354. doi: 10.1016/j.cellsig.2019.109354. Epub 2019 Jul 2. Cell Signal. 2019. PMID: 31271881
-
Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways.Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C554-C566. doi: 10.1152/ajpcell.00016.2022. Epub 2022 Feb 16. Am J Physiol Cell Physiol. 2022. PMID: 35171698 Free PMC article. Review.
Cited by
-
Angiostatic cues from the matrix: Endothelial cell autophagy meets hyaluronan biology.J Biol Chem. 2020 Dec 4;295(49):16797-16812. doi: 10.1074/jbc.REV120.014391. Epub 2020 Oct 5. J Biol Chem. 2020. PMID: 33020183 Free PMC article. Review.
-
Oncosuppressive functions of decorin.Mol Cell Oncol. 2015 Feb 25;2(3):e975645. doi: 10.4161/23723556.2014.975645. eCollection 2015 Jul-Sep. Mol Cell Oncol. 2015. PMID: 27308453 Free PMC article. Review.
-
The Role of Decorin Proteoglycan in Mitophagy.Cancers (Basel). 2022 Feb 4;14(3):804. doi: 10.3390/cancers14030804. Cancers (Basel). 2022. PMID: 35159071 Free PMC article. Review.
-
Exposed CendR Domain in Homing Peptide Yields Skin-Targeted Therapeutic in Epidermolysis Bullosa.Mol Ther. 2020 Aug 5;28(8):1833-1845. doi: 10.1016/j.ymthe.2020.05.017. Epub 2020 May 20. Mol Ther. 2020. PMID: 32497513 Free PMC article.
-
Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): a dual receptor antagonism.J Biol Chem. 2011 Jul 22;286(29):25947-62. doi: 10.1074/jbc.M111.243626. Epub 2011 May 19. J Biol Chem. 2011. PMID: 21596751 Free PMC article.
References
-
- Ramirez F., Rifkin D. B. (2003) Matrix Biol. 22, 101–107 - PubMed
-
- Bateman J. F., Boot-Handford R. P., Lamandé S. R. (2009) Nat. Rev. Genet. 10, 173–183 - PubMed
-
- Iozzo R. V., Murdoch A. D. (1996) FASEB J. 10, 598–614 - PubMed
-
- Ameye L., Young M. F. (2002) Glycobiology 12, 107R–116R - PubMed
-
- Iozzo R. V. (1999) J. Biol. Chem. 274, 18843–18846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

