β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK)
- PMID: 20974912
- PMCID: PMC2984171
- DOI: 10.1073/pnas.1009705107
β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK)
Abstract
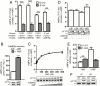
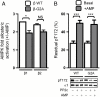
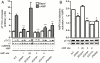
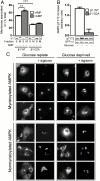
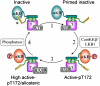
The AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that acts as a master metabolic regulator to maintain cellular energy balance following increased energy demand and increases in the AMP/ATP ratio. This regulation provides dynamic control of energy metabolism, matching energy supply with demand that is essential for the function and survival of organisms. AMPK is inactive unless phosphorylated on Thr172 in the α-catalytic subunit activation loop by upstream kinases (LKB1 or calcium-calmodulin-dependent protein kinase kinase β). How a rise in AMP levels triggers AMPK α-Thr172 phosphorylation and activation is incompletely understood. Here we demonstrate unequivocally that AMP directly stimulates α-Thr172 phosphorylation provided the AMPK β-subunit is myristoylated. Loss of the myristoyl group abolishes AMP activation and reduces the extent of α-Thr172 phosphorylation. Once AMPK is phosphorylated, AMP further activates allosterically but this activation does not require β-subunit myristoylation. AMP and glucose deprivation also promote membrane association of myristoylated AMPK, indicative of a myristoyl-switch mechanism. Our results show that AMP regulates AMPK activation at the initial phosphorylation step, and that β-subunit myristoylation is important for transducing the metabolic stress signal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. - PubMed
-
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. - PubMed
-
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. - PubMed
-
- Iseli TJ, et al. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280:13395–13400. - PubMed
-
- Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–1729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

