Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin
- PMID: 20974985
- PMCID: PMC3927452
- DOI: 10.4049/jimmunol.1000719
Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin
Abstract
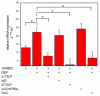
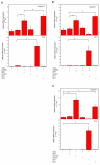
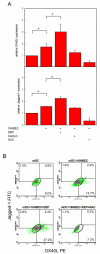
Ambient particulate matter, including diesel exhaust particles (DEP), promotes the development of allergic disorders. DEP increase oxidative stress and influence human bronchial epithelial cell (HBEC)-dendritic cell interactions via cytokines, including thymic stromal lymphopoietin (TSLP). Upregulation of TSLP results in Th2 responses. Using primary culture HBEC and human myeloid dendritic cell (mDC) cocultures, we show in this study that DEP upregulation of Th2 responses occurred via HBEC-dependent mechanisms that resulted from oxidative stress. Moreover, DEP-treated HBEC and ambient particulate matter-treated HBEC upregulated OX40 ligand (OX40L) and the Notch ligand Jagged-1 mRNA and expression on mDC. Upregulation of OX40L as well as Jagged-1 on mDC required HBEC and did not occur in the presence of N-acetylcysteine. Furthermore, OX40L and Jagged-1 upregulation was inhibited when HBEC expression of TSLP was silenced. Thus, DEP treatment of HBEC targeted two distinct pathways in mDC that were downstream of TSLP expression. Upregulation of OX40L and Jagged-1 by mDC resulted in mDC-driven Th2 responses. These studies expand our understanding of the mechanism by which ambient pollutants alter mucosal immunity and promote disorders such as asthma.
Figures





References
-
- Riedl MA. The effect of air pollution on asthma and allergy. Curr Allergy Asthma Rep. 2008;8:139–146. - PubMed
-
- Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. - PubMed
-
- Janssen NA, Brunekreef B, van Vliet P, Aarts F, Meliefste K, Harssema H, Fischer P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111:1512–1518. - PMC - PubMed
-
- Gergen PJ, Turkeltaub PC, Kovar MG. The prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 1987;80:669–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

