Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival
- PMID: 20975039
- PMCID: PMC2989777
- DOI: 10.1084/jem.20092241
Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival
Abstract
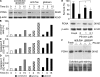
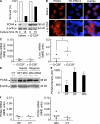
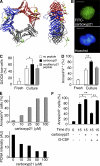
Neutrophil apoptosis is a highly regulated process essential for inflammation resolution, the molecular mechanisms of which are only partially elucidated. In this study, we describe a survival pathway controlled by proliferating cell nuclear antigen (PCNA), a nuclear factor involved in DNA replication and repairing of proliferating cells. We show that mature neutrophils, despite their inability to proliferate, express high levels of PCNA exclusively in their cytosol and constitutively associated with procaspases, presumably to prevent their activation. Notably, cytosolic PCNA abundance decreased during apoptosis, and increased during in vitro and in vivo exposure to the survival factor granulocyte colony-stimulating factor (G-CSF). Peptides derived from the cyclin-dependent kinase inhibitor p21, which compete with procaspases to bind PCNA, triggered neutrophil apoptosis thus demonstrating that specific modification of PCNA protein interactions affects neutrophil survival. Furthermore, PCNA overexpression rendered neutrophil-differentiated PLB985 myeloid cells significantly more resistant to TNF-related apoptosis-inducing ligand- or gliotoxin-induced apoptosis. Conversely, a decrease in PCNA expression after PCNA small interfering RNA transfection sensitized these cells to apoptosis. Finally, a mutation in the PCNA interdomain-connecting loop, the binding site for many partners, significantly decreased the PCNA-mediated antiapoptotic effect. These results identify PCNA as a regulator of neutrophil lifespan, thereby highlighting a novel target to potentially modulate pathological inflammation.
Figures



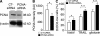

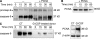
References
-
- Altznauer F., Martinelli S., Yousefi S., Thürig C., Schmid I., Conway E.M., Schöni M.H., Vogt P., Mueller C., Fey M.F., et al. 2004. Inflammation-associated cell cycle–independent block of apoptosis by survivin in terminally differentiated neutrophils. J. Exp. Med. 199:1343–1354 10.1084/jem.20032033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

